|
Neodymium Compounds
Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as Neodymium(III) chloride, NdCl3, Neodymium(III) sulfate, Nd2(SO4)3 and Neodymium acetate, Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as Neodymium(II) chloride, NdCl2 and Neodymium(II) iodide, NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.Burke M.W. (1996) Lighting II: Sources. In: Image Acquisition. Springer, Dordrecht. File:Neodymium tl1.jpg, Neodymium compounds in Fluorescent lamp, fluorescent tube light—from left to right, the sulfate, nitrate, and chloride File:Neodymium fluorescent1.jpg, Neodymium compounds in compact fluorescent lamp light File:Neodymium daylight1.jpg, Neodymium compounds in normal daylight Halides Neodymium can form four trihalides of the form NdX3. It reacts vigorously with all the stable halogens: : [a violet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a d-block element and a transition metal. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. Lutetium is a d-block element (thus also a transition metal), and on this basis its inclusion has been questioned; however, like its congeners scandium and yttrium in group 3, it behaves similarly to the other 14. The term rare-earth element or rare-earth metal is often used to include the stable group 3 elements Sc, Y, and Lu in addition to the 4f elements. All lanthanide elements form trivalent cations, Ln3+, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(II) Iodide
Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound. Neodymium(II) iodide is a violet solid. The compound is not stoichiometric. It melts at 562°C. Preparation Neodymium(II) iodide can be made by heating molten neodymium(III) iodide with neodymium metal at 800 and 580°C for 12 hours. It can also be obtained by reducing neodymium(III) iodide with neodymium in a vacuum at 800 to 900°C: :Nd + 2NdI3 → 3NdI2 The reaction of neodymium with mercury(II) iodide is also possible because neodymium is more reactive than mercury: :Nd + HgI2 → NdI2 + Hg Direct preparation from iodine and neodymium is also possible: :Nd + I2 → NdI2 The compound was first synthesized by John D. Corbett in 1961.Angelika Jungmann, R. Claessen, R. Zimmermann, G. e. Meng, P. Steiner, S. Hüfner, S. Tratzky, K. Stöwe, H. P. Beck: ''Photoemission of LaI2 and CeI2.'' In: ''Zeitschrift für Physik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Bicarbonate
Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia. Production Ammonium bicarbonate is produced by combining carbon dioxide and ammonia: : Since ammonium bicarbonate is thermally unstable, the reaction solution is kept cold, which allows the precipitation of the product as white solid. About 100,000 tons were produced in this way in 1997. Ammonia gas passed into a strong aqueous solution of the sesquicarbonate (a 2:1:1 mixture of (NH4)HCO3, (NH4)2CO3, and H2O) converts it into normal ammonium carbonate ((NH4)2CO3), which can be obtained in the crystalline condition from a solution prepared at about 30 °C. This compound on exposure to air gives off ammonia and reverts to ammonium bicarbonate. Salt of hartshorn Compositions containing ammonium carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Carbonate
Neodymium(III) carbonate is an inorganic compound, a salt, where neodymium is in the +3 oxidation state and the carbonate ion has charge −2.See https://pubchem.ncbi.nlm.nih.gov/compound/Neodymium_III_-carbonate-hydrate It has a chemical formula of Nd2(CO3)3. The anhydrous form is purple-red,Rare earth elements: Main volume, Phần 3 (Leopold Gmelin; Verlag Chemie, 1994), page 22; 68. Retrieved 4 February 2021. while the octahydrate is a pink solid.Handbook… (Pierre Villars, Karin Cenzual, Roman Gladyshevskii; Walter de Gruyter GmbH & Co KG, 24 thg 7, 2017 - 1970 pages), page 999. R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Oxalate
Neodymium(III) oxalate is the oxalate salt of neodymium, with the chemical formula of Nd2(C2O4)3 in the anhydrous or hydrate form. Its decahydrate decomposes to the anhydrous form when heated, and when heated further, decomposes to Nd2O2C2O4, finally obtaining neodymium(III) oxide. It dissolves in hydrochloric acid Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ... to form Nd(C2O4)Cl·3H2O.Moebius, R.; Matthes, F. The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. ''Zeitschrift für Chemie'', 1964. 4 (6): 234-235. ISSN: 0044-2402. References {{Oxalates Neodymium(III) compounds Oxalates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility Product
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent ''solubility product'' which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios. Definitions A solubility equilibrium exists when a chemical compound in the solid state is in chemical equilibrium with a solution containing the compound. This type of equilibrium is an example of dynamic equilibrium in that some individual molecules migrate between the solid and solution phases such that the rates of dissolution and precipitation are equal to one another. When equilibrium is established and the solid has not all dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Arsenate
Neodymium arsenate, also known as neodymium(III) arsenate, is the arsenate of neodymium with the chemical formula of NdAsO4. In this compound, neodymium exhibits the +3 oxidation state. It has good thermal stability, and its p''K''sp,c is 21.86±0.11. Preparation Neodymium arsenate can be obtained from the reaction between sodium arsenate (Na3AsO4) and neodymium chloride (NdCl3) in solution: : Na3AsO4 + NdCl3 → 3 NaCl + NdAsO4↓ When crystallizing from a lead pyroarsenate flux, neodymium arsenate crystals produced explode when cooled. Neodymium arsenate also occurs in nature as a mineral, Arsenoflorencite-(Nd). See also * Arsenic Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ... References Neodymium(III) compounds Arsenates {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Chloride
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA). Appearance NdCl3 is a mauve colored hygroscopic solid whose color changes to purple upon absorption of atmospheric water. The resulting hydrate, like many other neodymium salts, has the interesting property that it appears differe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Nitrate
Neodymium nitrate is an inorganic salt with the formula . It is typically encountered as the hexahydrate, Nd(NO3)3·6H2O, which is more accurately formulated as d(NO3)3(H2O)42H2O to reflect the crystal structure. It decomposes to NdONO3 at elevated temperature Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making .... It is used in the extraction and purification of neodymium from its ores. References Neodymium(III) compounds Nitrates {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
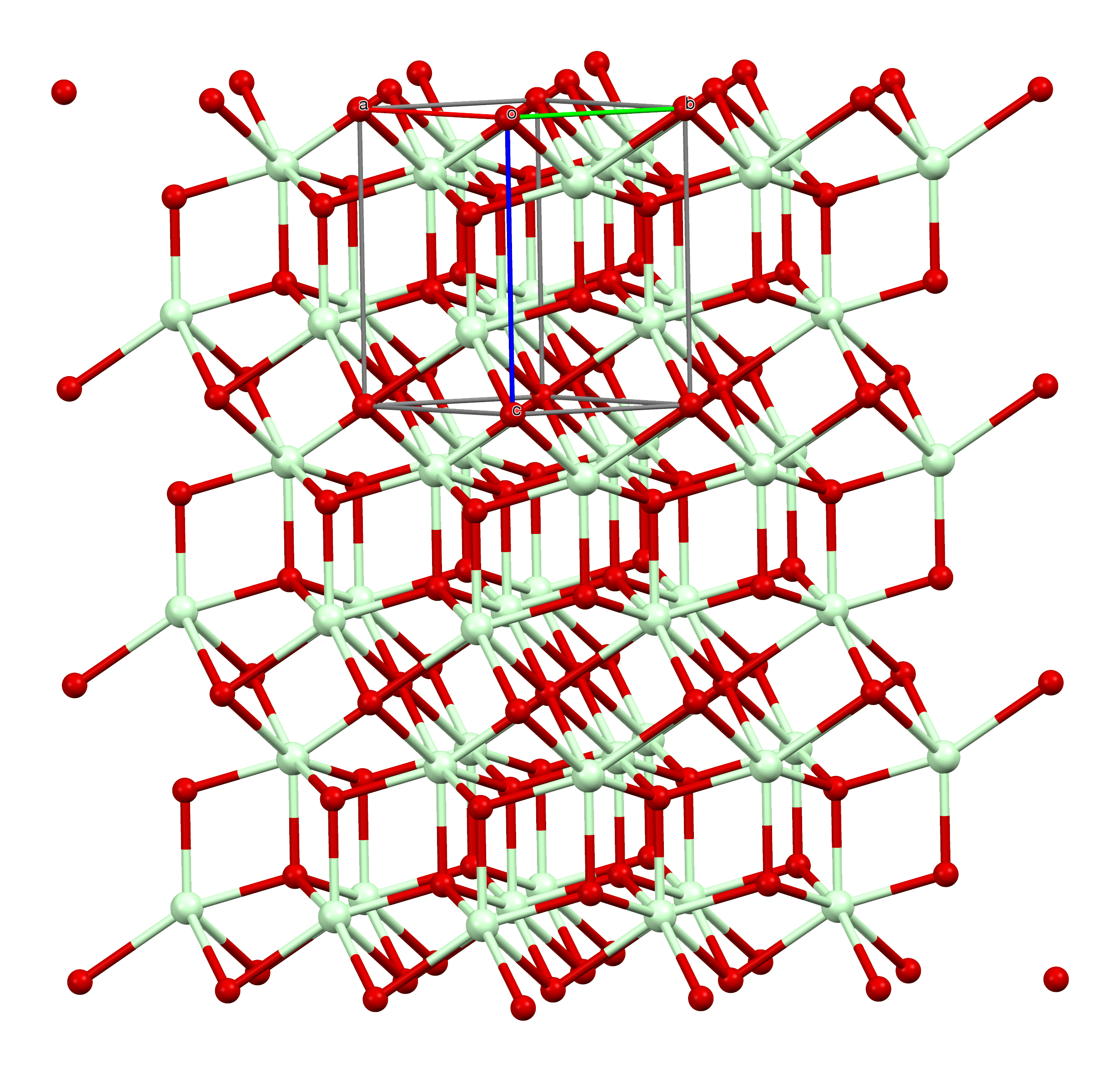
Neodymium(III) Oxide
Neodymium(III) oxide or neodymium sesquioxide is the chemical compound composed of neodymium and oxygen with the formula Nd2O3. It forms very light grayish-blue hexagonal crystals. The rare-earth mixture didymium, previously believed to be an element, partially consists of neodymium(III) oxide. Uses Neodymium(III) oxide is used to dope glass, including sunglasses, to make solid-state lasers, and to color glasses and enamels. Neodymium-doped glass turns purple due to the absorbance of yellow and green light, and is used in welding goggles. Some neodymium-doped glass is dichroic; that is, it changes color depending on the lighting. One kind of glass named for the mineral alexandrite appears blue in sunlight and red in artificial light. About 7000 tonnes of neodymium(III) oxide are produced worldwide each year. Neodymium(III) oxide is also used as a polymerization In polymer chemistry, polymerization (American English), or polymerisation (British English), is a proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |