|
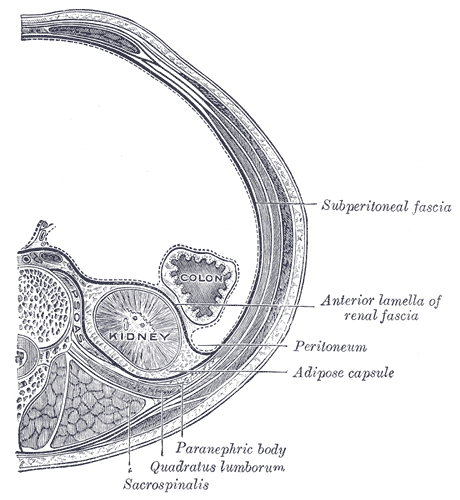
Kidney Anatomy
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder. The kidney participates in the control of the volume of various body fluids, fluid osmolality, acid-base balance, various electrolyte concentrations, and removal of toxins. Filtration occurs in the glomerulus: one-fifth of the blood volume that enters the kidneys is filtered. Examples of substances reabsorbed are solute-free water, sodium, bicarbonate, glucose, and amino acids. Examples of substances secreted are hydrogen, ammonium, potassium and uric acid. The nephron is the structural and func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retroperitoneal Space
The retroperitoneal space (retroperitoneum) is the spatium, anatomical space (sometimes a potential space) behind (''retro'') the peritoneum. It has no specific delineating anatomical structures. Organs are retroperitoneal if they have peritoneum on their anterior side only. Structures that are not suspended by mesentery in the abdominal cavity and that lie between the parietal peritoneum and abdominal wall are classified as retroperitoneal. This is different from organs that are not retroperitoneal, which have peritoneum on their posterior side and are suspended by mesentery in the abdominal cavity. The retroperitoneum can be further subdivided into the following: *Perirenal (or perinephric) space *Anterior pararenal (or paranephric) space *Posterior pararenal (or paranephric) space Retroperitoneal structures Structures that lie behind the peritoneum are termed "retroperitoneal". Organs that were once suspended within the abdominal cavity by mesentery but migrated posterior to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxins
A toxin is a naturally occurring poison produced by metabolic activities of living cells or organisms. They occur especially as proteins, often conjugated. The term was first used by organic chemist Ludwig Brieger (1849–1919), derived from '' toxic''. Toxins can be small molecules, peptides, or proteins that are capable of causing disease on contact with or absorption by body tissues interacting with biological macromolecules such as enzymes or cellular receptors. They vary greatly in their toxicity, ranging from usually minor (such as a bee sting) to potentially fatal even at extremely low doses (such as botulinum toxin). Terminology Toxins are often distinguished from other chemical agents strictly based on their biological origin. Less strict understandings embrace naturally occurring inorganic toxins, such as arsenic. Other understandings embrace synthetic analogs of naturally occurring organic poisons as toxins, and may or may not embrace naturally occurr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcitriol
Calcitriol is a hormone and the active form of vitamin D, normally made in the kidney. It is also known as 1,25-dihydroxycholecalciferol. It binds to and activates the vitamin D receptor in the nucleus of the cell, which then increases the expression of many genes. Calcitriol increases blood Calcium in biology, calcium mainly by increasing the uptake of calcium from the intestines. It can be given as a medication for the treatment of hypocalcemia, low blood calcium and hyperparathyroidism due to kidney disease, low blood calcium due to hypoparathyroidism, osteoporosis, osteomalacia, and familial hypophosphatemia, and can be taken by mouth or by intravenous, injection into a vein. Excessive amounts or intake can result in weakness, headache, nausea, constipation, urinary tract infections, and abdominal pain. Serious side effects may include high blood calcium and anaphylaxis. Calcitriol was identified as the active form of vitamin D in 1971 and the drug was approved for medic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin D
Vitamin D is a group of structurally related, fat-soluble compounds responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, along with numerous other biological functions. In humans, the most important compounds within this group are vitamin D3 ( cholecalciferol) and vitamin D2 ( ergocalciferol). Unlike the other twelve vitamins, vitamin D is only conditionally essential, as with adequate skin exposure to the ultraviolet B (UVB) radiation component of sunlight there is synthesis of cholecalciferol in the lower layers of the skin's epidermis. For most people, skin synthesis contributes more than diet sources. Vitamin D can also be obtained through diet, food fortification and dietary supplements. In the U.S., cow's milk and plant-based milk substitutes are fortified with vitamin D3, as are many breakfast cereals. Government dietary recommendations typically assume that all of a person's vitamin D is taken by mouth, given the potential for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nephron
The nephron is the minute or microscopic structural and functional unit of the kidney. It is composed of a renal corpuscle and a renal tubule. The renal corpuscle consists of a tuft of capillaries called a glomerulus and a cup-shaped structure called Bowman's capsule. The renal tubule extends from the capsule. The capsule and tubule are connected and are composed of epithelial cells with a lumen. A healthy adult has 1 to 1.5 million nephrons in each kidney. Blood is filtered as it passes through three layers: the endothelial cells of the capillary wall, its basement membrane, and between the podocyte foot processes of the lining of the capsule. The tubule has adjacent peritubular capillaries that run between the descending and ascending portions of the tubule. As the fluid from the capsule flows down into the tubule, it is processed by the epithelial cells lining the tubule: water is reabsorbed and substances are exchanged (some are added, others are removed); first wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uric Acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the Chemical formula, formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. Hyperuricemia, High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones. Chemistry Uric acid was first isolated from kidney stones in 1776 by Swedish chemist Carl Wilhelm Scheele. In 1882, the Ukrainian chemist Ivan Horbaczewski first synthesized uric acid by melting urea with glycine. Uric acid displays lactam–lactim tautomerism. Uric acid crystallizes in the lactam form, with computational chemistry also indicating that tautomer to be the most stable. Uric acid is a diprotic acid with pKa, p''K''a1 = 5.4 and p''K''a2 =&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create cation, an ion with a positive charge (which combines with anions to form salts). In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-flame color, colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by Organic compound, organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it. Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted–Lowry acid–base theory, Brønsted acids (proton donors): : The ammonium ion is mildly acidic, reacting with Brønsted bases to return ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living Organism, organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is -glucose, while its Stereoisomerism, stereoisomer L-glucose, -glucose is produced synthetically in comparatively small amounts and is less biologicall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid (). The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid (); and the conjugate acid of , t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |