|
Isopropenyl Compounds
In organic chemistry, 1-propenyl (or simply propenyl) has the formula CH=CHCH3 and 2-propenyl (isopropenyl) has the formula CH2=C-CH3. These groups are found in many compounds. Propenyl compounds are Isomer , isomeric with allyl compounds, which have the formula CH2-CH=CH2. Chemicals with 1-propenyl groups *2-Chloropropylene, 2-chloropropylene *propenylbenzene (β-methylstyrene). Many phenylpropanoids and their derivatives feature derivatives of propenylbenzene: *Anethole *Asarone *Carpacin *Coniferyl alcohol *Isoeugenol *Isosafrole *Methyl isoeugenol *Pseudoisoeugenol Chemicals with 2-propenyl groups Isopropenyl acetate is a 2-propenyl ester, synthesizable from ketene. Several terpenes feature 2-propenyl substituents: *carvone *limonene See also * Propene * Functional group References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Isoeugenol
Methyl isoeugenol (isomethyleugenol) is a phenylpropanoid, the methyl ether of isoeugenol, found in certain essential oils. It can occur as both (''E'')- and (''Z'')-isomers. See also * Methyl eugenol Methyl eugenol (allylveratrol) is a natural chemical compound classified as a phenylpropene, a type of phenylpropanoid. It is the methyl ether of eugenol and is important to insect behavior and pollination. It is found in various essential oils. ... References O-methylated phenylpropanoids Phenylpropenes {{Ether-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like odor. Propylene is a product of combustion from forest fires, cigarette smoke, and motor vehicle and aircraft exhaust. It was discovered in 1850 by A. W. von Hoffmann's student Captain (later Major General) John Williams Reynolds as the only gaseous product of thermal decomposition of amyl alcohol to react with chlorine and bromine. Production Steam cracking The dominant technology for producing propylene is steam cracking, using propane as the feedstock. Cracking propane yields a mixture of ethylene, propylene, methane, hydrogen gas, and other related compounds. The yield of propylene is about 15%. The other principal feedstock is naphtha, especially in the Middle East and Asia. Propylene can be separated by fractional distilla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limonene
Limonene () is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the essential oil of citrus fruit peels. The (+)-isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common (−)-isomer has a piny, turpentine-like odor, and is found in the edible parts of such plants as caraway, dill, and bergamot orange plants. Limonene takes its name from Italian ''limone'' ("lemon"). Limonene is a chiral molecule, and biological sources produce one enantiomer: the principal industrial source, citrus fruit, contains (+)-limonene (''d''-limonene), which is the (''R'')-enantiomer. (+)-Limonene is obtained commercially from citrus fruits through two primary methods: centrifugal separation or steam distillation. In plants (+)-Limonene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (''Carum carvi''), spearmint (''Mentha spicata''), and dill. Uses Food applications Both carvones are used in the food and flavor industry. As the compound most responsible for the flavor of caraway, dill, and spearmint, carvone has been used for millennia in food. Food applications are mainly met by carvone made from limonene. ''R''-(−)-Carvone is also used for air freshening products and, like many essential oils, oils containing carvones are used in aromatherapy and alternative medicine. Agriculture ''S''-(+)-Carvone is also used to prevent premature sprouting of potatoes during storage, being marketed in the Netherlands for this purpose under the name ''Talent''. Insect control ''R''-(−)-Carvone has been approved by the U.S. Environmental Protection Agency for use as a mosquito repellent. Stereoiso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly Pinophyta, conifers. In plants, terpenes and terpenoids are important mediators of ecological biological interaction, interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control. Terpenes are classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine. The one terpene that has major applications is natural rubber (i.e., polyisoprene). The possibility that other terpenes could be used as precursors to pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
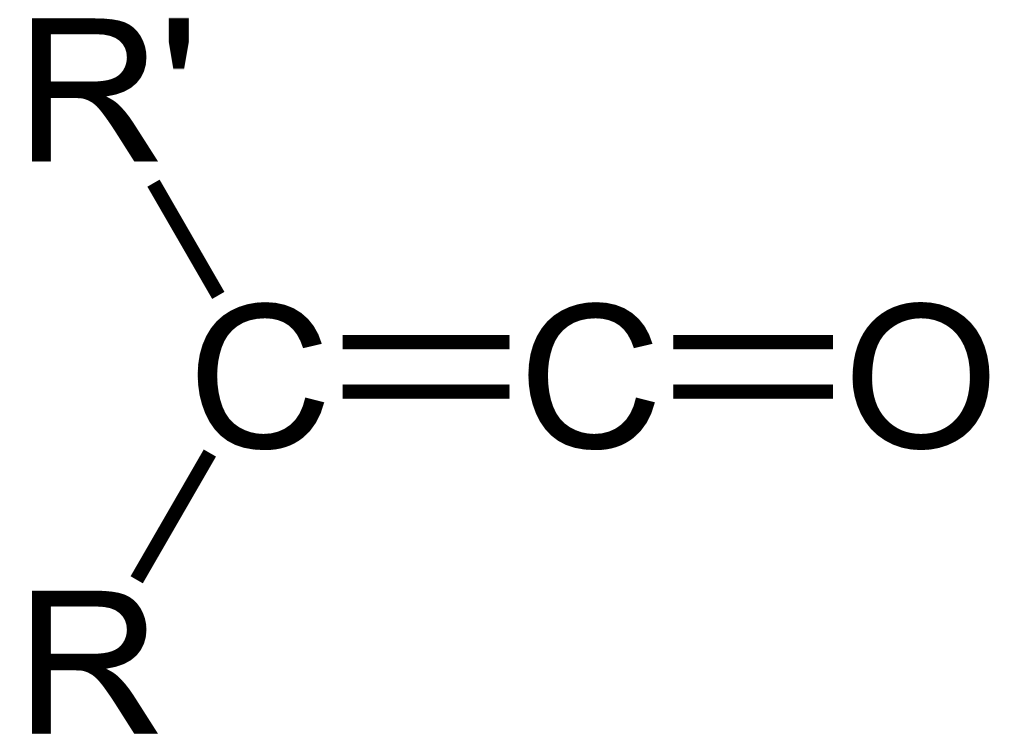
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropenyl Acetate
Isopropenyl acetate is an organic compound, which is the acetate ester of the enol tautomer of acetone. This colorless liquid is significant commercially as the principal precursor to acetylacetone. In organic synthesis, it is used to prepare enol acetates of ketones and acetonides from diols. Preparation Isopropenyl acetate is prepared by treating acetone with ketene In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The na .... Upon heating over a metal surface, isopropenyl acetate rearranges to acetylacetone. Reactions Isopropenyl acetate is used to prepare other isopropenyl ethers. Isopropenyl acetate reacts with ketones to give new enol acetates: :{{chem2, CH2\dCH(OAc)CH3 + RC(O)CH3 -> CH2\dCH(OAc)R + (CH3)2C\dO References Acetate esters Isopropenyl esters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P-isopropenylphenol
4-Isopropenylphenol is an organic compound with the formula . The molecule consists of a 2-propenyl group (CH2=''C''-CH3) affixed to the 4 position of phenol. The compound is an intermediate in the production of bisphenol A (BPA), 2.7 Mkg/y of which are produced annually (2007). It is also generated by the recycling of ''o,p''-BPA, a byproduct of the production of the ''p,p''-isomer of BPA. Synthesis and reactions The high-temperature hydrolysis of BPA gives the title compound together with phenol: : The compound can also be produced by catalytic dehydrogenation of 4-isopropylphenol. 4-Isopropenylphenol undergoes ''O''-protonation by sulfuric acid, giving the carbocation Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ..., which undergoes a variety of dimerization reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudoisoeugenol
Pseudoisoeugenol is a naturally occurring phenylpropene and an isomer of eugenol. Natural occurrence and derivatives Pseudoisoeugenol naturally occurs in the essential oils of roots from plants within the genus ''Pimpinella''. In addition to its standard form, the compound also occurs in a variety of structural derivatives. Common derivatives include the compound with its side chain bearing an epoxide functional group and the aromatic ring being associated with one of many possible esters in the 2nd position. Common esters of the phenol group include angelic acid, 2-methylbutanoic acid, tiglic acid, and 2-methylpropionic acid esters. Hydrolysis of these esters, either ''in vivo'' or by using strong acids, forms 2-methyl-5-methoxybenzofuran. Biosynthesis Biosynthesis of the compound is hypothesized to proceed via a NIH shift of anethole Anethole (also known as anise camphor) is an organic compound that is widely used as a flavoring substance. It is a derivative of the aroma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isosafrole
Isosafrole is an organic compound that is used in the fragrance industry. Structurally, the molecule is related to allylbenzene, a type of aromatic organic chemical. Its fragrance is reminiscent of anise or licorice. It is found in small amounts in various essential oils, but is most commonly obtained by isomerizing the plant oil safrole. It exists as two geometric isomers, ''cis''-isosafrole and ''trans''-isosafrole. Isosafrole is a precursor to the important fragrance piperonal.Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe and Horst Surburg "Flavors and Fragrances" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2003. It can also be converted via the intermediate compound MDP2P into the psychoactive drug MDMA 3,4-Methylenedioxymethamphetamine (MDMA), commonly known as ecstasy (tablet form), and molly (crystal form), is an empathogen–entactogenic dru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |