|
Ice Hockey Teams In Quebec
Ice is water that is frozen into a solid state, typically forming at or below temperatures of 0 ° C, 32 ° F, or 273.15 K. It occurs naturally on Earth, on other planets, in Oort cloud objects, and as interstellar ice. As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color. Virtually all of the ice on Earth is of a hexagonal crystalline structure denoted as ''ice Ih'' (spoken as "ice one h"). Depending on temperature and pressure, at least nineteen phases ( packing geometries) can exist. The most common phase transition to ice Ih occurs when liquid water is cooled below (, ) at standard atmospheric pressure. When water is cooled rapidly (quenching), up to three types of amorphous ice can form. Interstellar ice is overwhelmingly low-density amorphous ice (LDA), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kilogram-force
The kilogram-force (kgf or kgF), or kilopond (kp, from ), is a non-standard Gravitational metric system, gravitational metric unit of force. It is not accepted for use with the International System of Units (SI) and is deprecated for most uses. The kilogram-force is equal to the magnitude of the force exerted on one kilogram of mass in a gravitational field (standard gravity, a conventional value approximating the average magnitude of gravity on Earth). That is, it is the weight of a kilogram under standard gravity. One kilogram-force is defined as .NIST]''Guide for the Use of the International System of Units (SI)''Special Publication 811, (1995) page 51 Similarly, a gram-force is , and a milligram-force is . History The gram-force and kilogram-force were never well-defined units until the CGPM adopted a ''standard acceleration of gravity'' of 9.80665 m/s2 for this purpose in 1901, though they had been used in low-precision measurements of force before that time. Even then, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
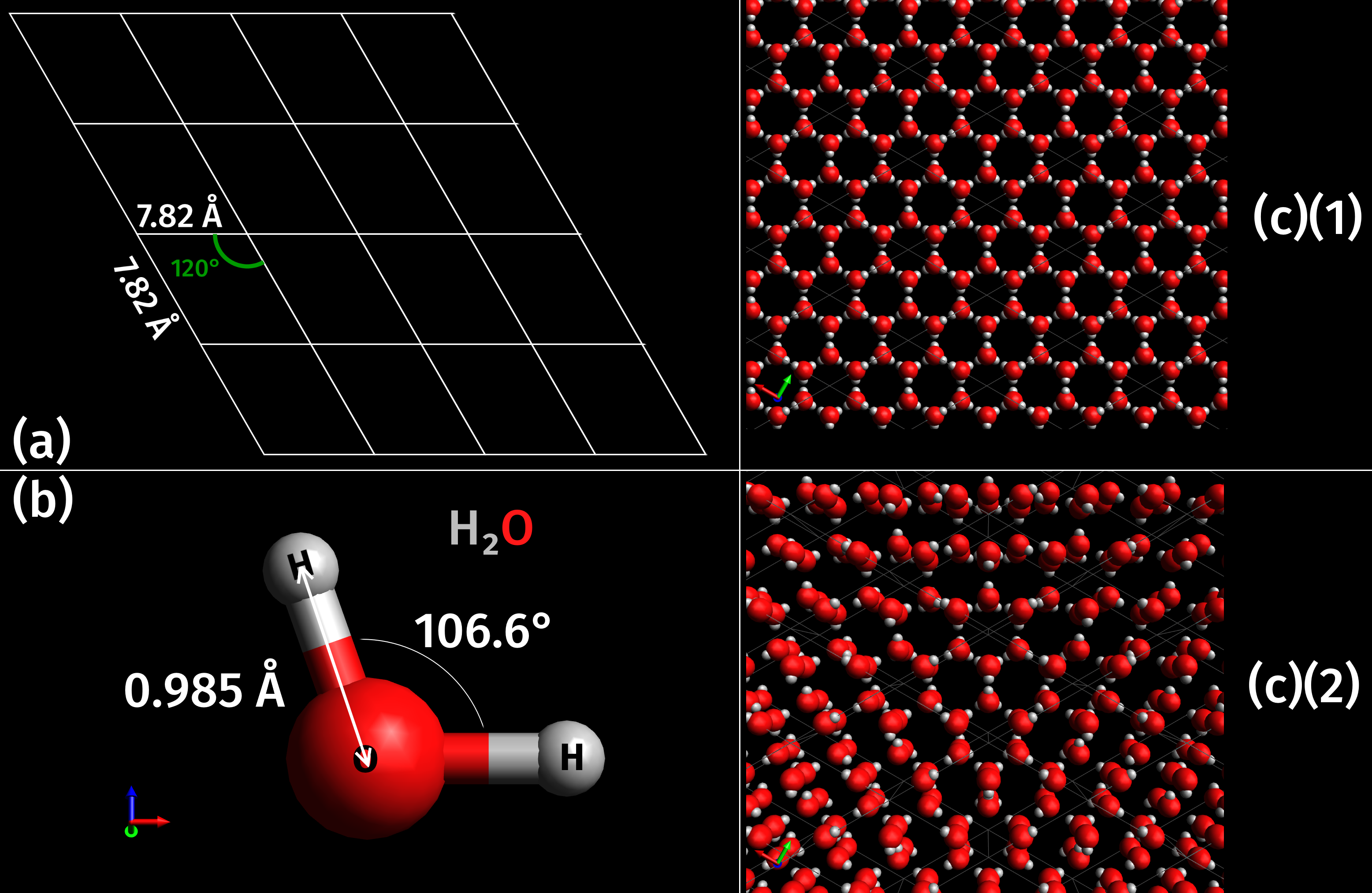
Phases Of Ice
Variations in pressure and temperature give rise to different phases of ice, which have varying properties and molecular geometries. Currently, twenty-one phases, including both crystalline and Amorphous solid, amorphous ices have been observed. In modern history, phases have been discovered through scientific research with various techniques including pressurization, force application, nucleation agents, and others. On Earth, most ice is found in the hexagonal Ice Ih phase. Less common phases may be found in the atmosphere and underground due to more extreme pressures and temperatures. Some phases are manufactured by humans for nano scale uses due to their properties. In space, amorphous ice is the most common form as confirmed by observation. Thus, it is theorized to be the most common phase in the universe. Various other phases could be found naturally in astronomical objects. Theory Most liquids under increased pressure freeze at ''higher'' temperatures because the pressu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |