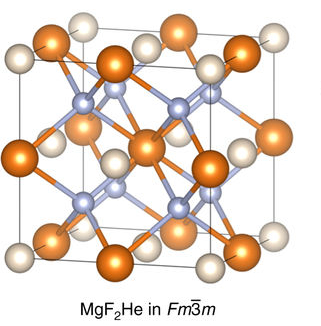
|
Helium Compounds
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. Helium is a very hard atom with a Pearson hardness of 12.3 eV. It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point (4.2 K) of any known substance. Repulsive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the Chemical element, elements, and it does not have a melting point at standard pressures. It is the second-lightest and second-most Abundance of the chemical elements, abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Impact
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of the first ionization techniques developed for mass spectrometry. However, this method is still a popular ionization technique. This technique is considered a hard (high fragmentation) ionization method, since it uses highly energetic electrons to produce ions. This leads to extensive fragmentation, which can be helpful for structure determination of unknown compounds. EI is the most useful for organic compounds which have a molecular weight below 600 amu. Also, several other thermally stable and volatile compounds in solid, liquid and gas states can be detected with the use of this technique when coupled with various separation methods. History Electron ionization was first described in 1918 by Canadian-American Physicist Arthur J. Dem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
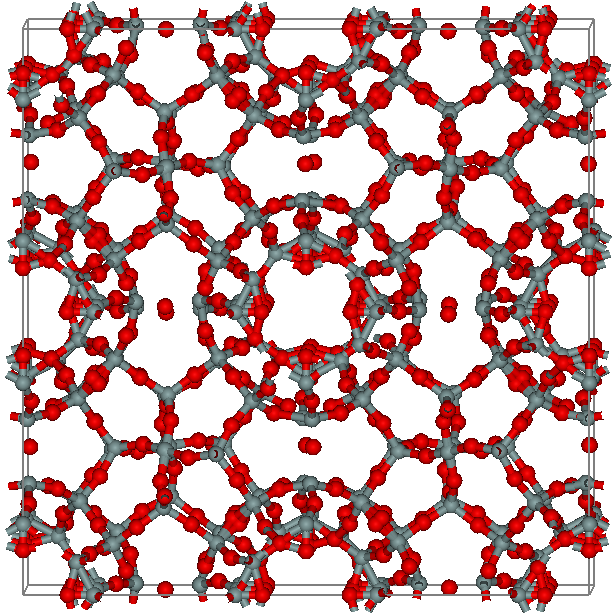
Chibaite
Chibaite is a rare silicate mineral. It is a silica clathrate with formula (n = 3/17 (max)). The mineral is cubic (diploidal class, m) and the silica hosts or traps various hydrocarbon molecules, such as methane, ethane, propane and isobutane.Chibaite on Mindat.org Chibaite was first described for specimens collected from Arakawa, Minamibōsō, Chiba Prefecture, Honshu Island, |
Melanophlogite
Melanophlogite (MEP) is a rare silicate mineral and a polymorph of silica (SiO2). It has a zeolite-like porous structure which results in relatively low and not well-defined values of its density and refractive index. Melanophlogite often overgrows crystals of sulfur or calcite and typically contains a few percent of organic and sulfur compounds. Darkening of organics in melanophlogite upon heating is a possible origin of its name, which comes from the Greek for "black" and "to be burned". History Melanophlogite was identified and named by Arnold von Lasaulx in 1876 although G. Alessi had described a very similar mineral as early as in 1827. The mineral had a cubic crystal structure; chemical analysis revealed that it is mainly composed of SiO2, but also contains up to 12% of carbon and sulfur. It was suggested that the decomposition of organic matter (carbon) in the mineral was responsible for its blackening upon heating. All studied samples originated from Sicily, and thus the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described as having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference" stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electride
An electride is an ionic compound in which an electron serves the role of the anion. Solutions Solutions of alkali metals in ammonia are electride salts. In the case of sodium, these blue solutions consist of a(NH3)6sup>+ and solvated electrons: :Na + 6 NH3 → a(NH3)6sup>+ + e− The cation a(NH3)6sup>+ is an octahedral coordination complex. Despite the name, the electron does not leave the sodium-ammonia complex, but it is transferred from Na to the vacant orbitals of the coordinated ammonia molecules. Similar solutions exist in hexamethylphosphoramide. Solid salts Many "inorganic electrides" have been described. Addition of a complexant like crown ether or .2.2cryptand to a solution of a(NH3)6sup>+e− affords a (crown ether)sup>+e− or a(2,2,2-crypt)sup>+e−. Evaporation of these solutions yields a blue-black paramagnetic solid with the formula a(2,2,2-crypt)sup>+e−. Most solid electride salts decompose above 240 K, although a24Al28O64sup>4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
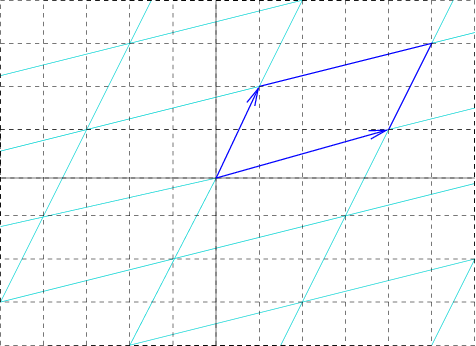
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector In mathematics, a unit vector in a normed vector space is a Vector (mathematics and physics), vector (often a vector (geometry), spatial vector) of Norm (mathematics), length 1. A unit vector is often denoted by a lowercase letter with a circumfle ..., for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
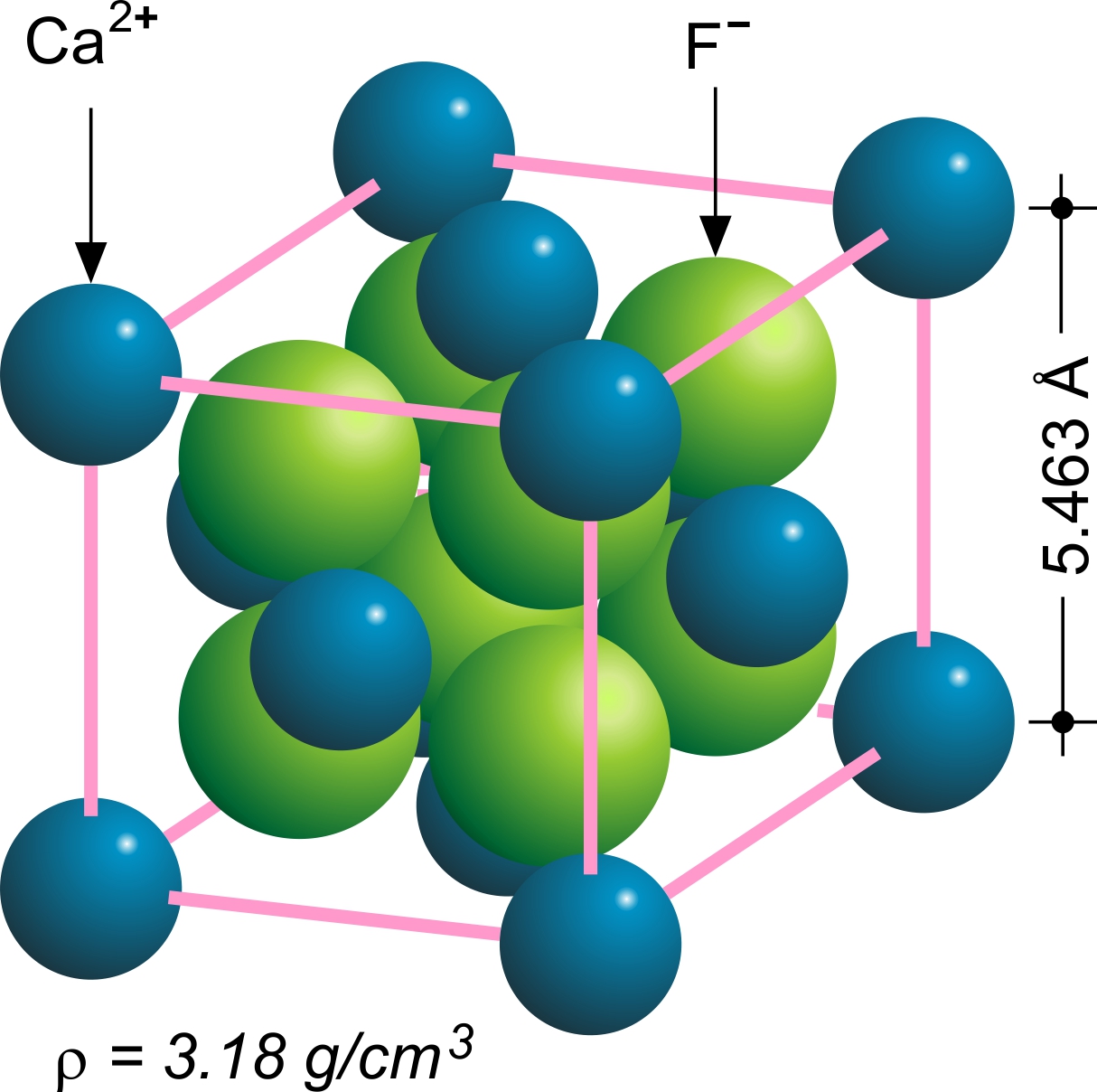
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc) Note: the term fcc is often used in synonym for the ''cubic close-packed'' or ccp structure occurring in metals. However, fcc stands for a face-centered cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed. Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais latices ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamically Stable
In chemistry, chemical stability is the thermodynamic stability of a chemical system, in particular a chemical compound or a polymer. Colloquially, it may instead refer to kinetic persistence, the shelf-life of a metastable substance or system; that is, the timescale over which it begins to degrade. Thermodynamic stability occurs when a system is in its lowest energy state, or in chemical equilibrium with its environment. This may be a dynamic equilibrium in which individual atoms or molecules change form, but their overall number in a particular form is conserved. This type of chemical thermodynamic equilibrium will persist indefinitely unless the system is changed. Chemical systems might undergo changes in the phase of matter or a set of chemical reactions. State A is said to be more thermodynamically stable than state B if the Gibbs free energy of the change from A to B is positive. Versus reactivity Thermodynamic stability applies to a particular system. The reactivity of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |