|
Good Clinical Practice
In drug development and production, good clinical practice (GCP) is an international quality standard, which governments can then transpose into regulations for clinical trials involving human subjects. GCP follows the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), and enforces tight guidelines on ethical aspects of clinical research. High standards are required in terms of comprehensive documentation for the clinical protocol, record keeping, training, and facilities, including computers and software. Quality assurance and inspections ensure that these standards are achieved. GCP aims to ensure that the studies are scientifically authentic and that the clinical properties of the investigational product are properly documented. GCP guidelines include protection of human rights for the subjects and volunteers in a clinical trial. It also provides assurance of the safety and efficacy of the newly develope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. These h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
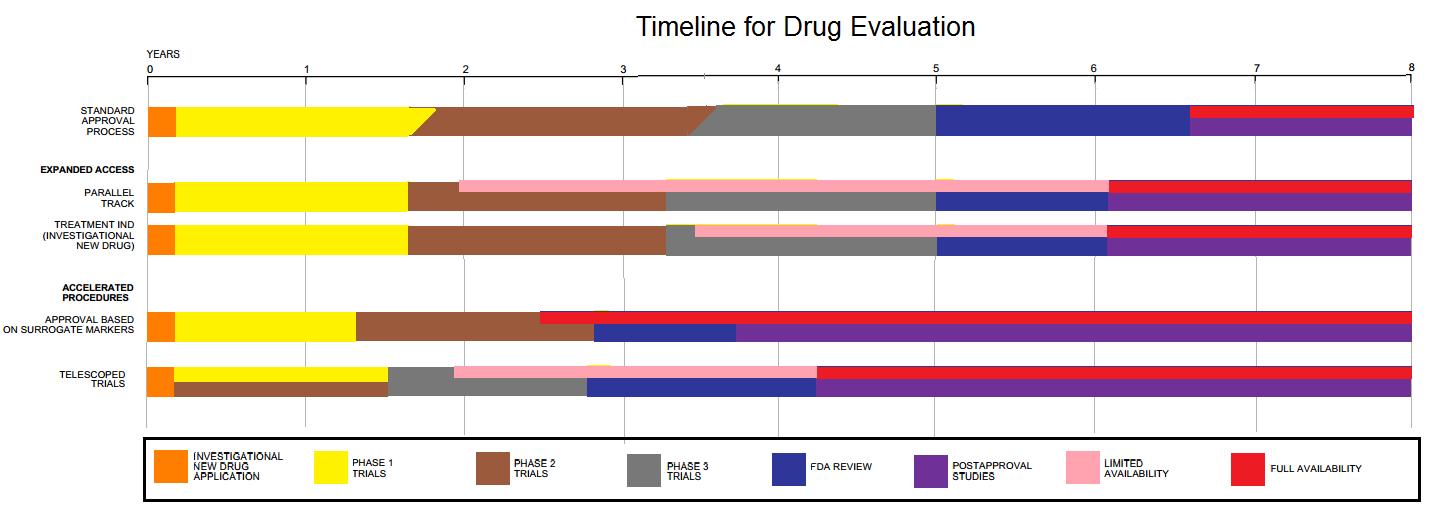
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Research
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness (efficacy) and safety of medications, devices, diagnostic products, and treatment regimens intended for improving human health. These research procedures are designed for the prevention, treatment, diagnosis or understanding of disease symptoms. Clinical research is different from clinical practice: in clinical practice, established treatments are used to improve the condition of a person, while in clinical research, evidence is collected under rigorous study conditions on groups of people to determine the efficacy and safety of a treatment. Description The term "clinical research" refers to the entire process of studying and writing about a drug, a medical device or a form of treatment, which includes conducting interventional studies (clinical trials) or observational studies on human participants. Clinical research can cover any medical method or product from it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Good Clinical Practice
In drug development and production, good clinical practice (GCP) is an international quality standard, which governments can then transpose into regulations for clinical trials involving human subjects. GCP follows the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), and enforces tight guidelines on ethical aspects of clinical research. High standards are required in terms of comprehensive documentation for the clinical protocol, record keeping, training, and facilities, including computers and software. Quality assurance and inspections ensure that these standards are achieved. GCP aims to ensure that the studies are scientifically authentic and that the clinical properties of the investigational product are properly documented. GCP guidelines include protection of human rights for the subjects and volunteers in a clinical trial. It also provides assurance of the safety and efficacy of the newly develope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Good Laboratory Practice
The Principles of Good Laboratory Practice (GLP) establish rules and criteria for a quality system that oversees the organizational processes and conditions in which non-clinical (non-pharmaceutical) health and environmental safety–or simply toxicology–studies are planned, conducted, monitored, recorded, reported, and archived. These principles apply to the toxicity testing of chemicals in commerce, to ensure the quality and integrity of the safety data submitted by manufacturers to regulatory authorities globally. History The historical events leading to the proposal of the Good Laboratory Practice (GLP) regulations are crucial for understanding why these regulations are important to improve the quality and integrity of chemical safety data. They were developed in response to concerns about the reliability of toxicity data from industry. The GLP regulations aim to standardize procedures and practices to ensure accurate, reliable, and traceable safety data. GLP was first introd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ben Goldacre
Ben Michael Goldacre (born 20 May 1974) is a British physician, academic and science writer. He is the first Bennett Professor of Evidence-Based Medicine and director of the Bennett Institute for Applied Data Science at the University of Oxford. He is a founder of the AllTrials campaign and OpenTrials, aiming to require open science practices in clinical trials. Goldacre is known in particular for his ''Bad Science'' column in ''The Guardian'', which he wrote between 2003 and 2011, and is the author of four books: '' Bad Science'' (2008), a critique of irrationality and certain forms of alternative medicine; '' Bad Pharma'' (2012), an examination of the pharmaceutical industry, its publishing and marketing practices, and its relationship with the medical profession; ''I Think You'll Find It's a Bit More Complicated Than That'', a collection of his journalism; and ''Statins'', about evidence-based medicine. Goldacre frequently delivers free talks about bad science; he describes h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bad Pharma
''Bad Pharma: How Drug Companies Mislead Doctors and Harm Patients'' is a book by the British physician and academic Ben Goldacre about the pharmaceutical industry, its relationship with the medical profession, and the extent to which it controls academic research into its own products.Luisa Dillner"Bad Pharma by Ben Goldacre – review" ''The Guardian'', 17 October 2012. It was published in the UK in September 2012 by the Fourth Estate imprint of HarperCollins, and in the United States in February 2013 by Faber and Faber. Goldacre argues in the book that "the whole edifice of medicine is broken", because the evidence on which it is based is systematically distorted by the pharmaceutical industry. He writes that the industry finances most of the clinical trials into its own products and much of doctors' continuing education, that clinical trials are often conducted on small groups of unrepresentative subjects and negative data is routinely withheld, and that apparently independe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Declaration Of Helsinki
The Declaration of Helsinki (DoH, ) is a set of ethical principles regarding human experimentation developed originally in 1964 for the medical community by the World Medical Association (WMA). It is widely regarded as the cornerstone document on human research ethics. It is not a legally binding instrument under international law, but instead draws its authority from the degree to which it has been codified in, or influenced, national or regional legislation and regulations. Its role was described by a Brazilian forum in 2000 in these words: "Even though the Declaration of Helsinki is the responsibility of the World Medical Association, the document should be considered the property of all humanity." Principles The Declaration is morally binding on physicians, and that obligation overrides any national or local laws or regulations, if the Declaration provides for a higher standard of protection of humans than the latter. Investigators still have to abide by local legislation b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial Protocol
In natural and social science research, a protocol is most commonly a predefined procedural method in the design and implementation of an experiment. Protocols are written whenever it is desirable to standardize a laboratory method to ensure successful replication of results by others in the same laboratory or by other laboratories. Additionally, and by extension, protocols have the advantage of facilitating the assessment of experimental results through peer review. In addition to detailed procedures, equipment, and instruments, protocols will also contain study objectives, reasoning for experimental design, reasoning for chosen sample sizes, safety precautions, and how results were calculated and reported, including statistical analysis and any rules for predefining and documenting excluded data to avoid bias. Similarly, a protocol may refer to the procedural methods of health organizations, commercial laboratories, manufacturing plants, etc. to ensure their activities (e.g., ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Institutional Review Board
An institutional review board (IRB), also known as an independent ethics committee (IEC), ethical review board (ERB), or research ethics board (REB), is a committee at an institution that applies research ethics by reviewing the methods proposed for research involving human subjects, to ensure that the projects are ethical. The main goal of IRB reviews is to ensure that study participants are not harmed (or that harms are minimal and outweighed by research benefits). Such boards are formally designated to approve (or reject), monitor, and review biomedical and behavioral research involving humans, and they are legally required in some countries under certain specified circumstances. Most countries use some form of IRB to safeguard ethical conduct of research so that it complies with national and international norms, regulations or codes. The purpose of the IRB is to assure that appropriate steps are taken to protect the rights and welfare of people participating in a research stu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Institutes Of Health
The National Institutes of Health (NIH) is the primary agency of the United States government responsible for biomedical and public health research. It was founded in 1887 and is part of the United States Department of Health and Human Services (HHS). Many NIH facilities are located in Bethesda, Maryland, and other nearby suburbs of the Washington metropolitan area, with other primary facilities in the Research Triangle Park in North Carolina and smaller satellite facilities located around the United States. The NIH conducts its scientific research through the NIH Intramural Research Program (IRP) and provides significant biomedical research funding to non-NIH research facilities through its Extramural Research Program. , the IRP had 1,200 principal investigators and more than 4,000 postdoctoral fellows in basic, translational, and clinical research, being the largest biomedical research institution in the world, while, as of 2003, the extramural arm provided 28% of biomedical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |