|
Drug Safety
Pharmacovigilance (PV, or PhV), also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: (Greek for drug) and (Latin for to keep watch). As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended. That definition includes lack of efficacy: that means that the doses normally used for prevention, diagnosis, or treatment of a disease—or, especially in the case of device, for the modification of physiological disorder function. In 2010, the European Union expanded PV to include medication errors such as overdose, misuse, and abuse of a drug as well as drug exposure during pregnancy and breastfeeding. These are monitored even in the absence of an adverse event, because they may result in an adver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Science
Pharmacy is the science and practice of discovering, producing, preparing, dispensing, reviewing and monitoring medications, aiming to ensure the safe, effective, and affordable use of medication, medicines. It is a miscellaneous science as it links health sciences with pharmaceutical sciences and natural sciences. The professional practice is becoming more clinically oriented as most of the drugs are now manufactured by pharmaceutical industries. Based on the setting, pharmacy practice is either classified as community or institutional pharmacy. Providing direct patient care in the community of institutional pharmacies is considered clinical pharmacy. The scope of pharmacy practice includes more traditional roles such as compounding and dispensing of medications. It also includes more modern services related to health care including clinical services, reviewing medications for safety and efficacy, and providing drug information with patient counselling. Pharmacists, therefore, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Efficacy
Efficacy is the ability to perform a task to a satisfactory or expected degree. The word comes from the same roots as '' effectiveness'', and it has often been used synonymously, although in pharmacology a distinction is now often made between efficacy and effectiveness. The word ''efficacy'' is used in pharmacology and medicine to refer both to the maximum response achievable from a pharmaceutical drug in research settings, and to the capacity for sufficient therapeutic effect or beneficial change in clinical settings. Pharmacology In pharmacology, efficacy () is the maximum response achievable from an applied or dosed agent, for instance, a small molecule drug. Intrinsic activity is a relative term for a drug's efficacy relative to a drug with the highest observed efficacy. It is a purely descriptive term that has little or no mechanistic interpretation. In order for a drug to have an effect, it needs to bind to its target, and then to affect the function of this tar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Risk Management
Risk management is the identification, evaluation, and prioritization of risks, followed by the minimization, monitoring, and control of the impact or probability of those risks occurring. Risks can come from various sources (i.e, Threat (security), threats) including uncertainty in Market environment, international markets, political instability, dangers of project failures (at any phase in design, development, production, or sustaining of life-cycles), legal liabilities, credit risk, accidents, Natural disaster, natural causes and disasters, deliberate attack from an adversary, or events of uncertain or unpredictable root cause analysis, root-cause. Retail traders also apply risk management by using fixed percentage position sizing and risk-to-reward frameworks to avoid large drawdowns and support consistent decision-making under pressure. There are two types of events viz. Risks and Opportunities. Negative events can be classified as risks while positive events are classifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Qualified Person For Pharmacovigilance
{{Distinguish, Qualified person (European Union) In the European Union, the Qualified Person Responsible For Pharmacovigilance (QPPV) is an individual, usually an employee of a pharmaceutical company, who is personally responsible for the safety of the human pharmaceutical products marketed by that company in the EU. This function was established in 2004 by article 23 of regulation (EC) No 726/2004. The article establishes that the holder of a marketing authorization for a drug for human use must have a QPPV. When a company submits an application for permission to bring a medicinal product onto the market, the company submits a description of its system for monitoring the safety of the product in actual use (a pharmacovigilance system) and proof that the services of a QPPV are in place. "The holder of an authorisation for a medicinal product for human use granted in accordance with the provisions of this Regulation shall have permanently and continuously at his disposal an appropria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Geneva, Switzerland, and has 6 regional offices and 150 field offices worldwide. Only sovereign states are eligible to join, and it is the largest intergovernmental health organization at the international level. The WHO's purpose is to achieve the highest possible level of health for all the world's people, defining health as "a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity." The main functions of the World Health Organization include promoting the control of epidemic and endemic diseases; providing and improving the teaching and training in public health, the medical treatment of disease, and related matters; and promoting the establishment of international standards for biologic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Common Terminology Criteria For Adverse Events
The Common Terminology Criteria for Adverse Events (CTCAE), formerly called the Common Toxicity Criteria (CTC or NCI-CTC), are a set of criteria for the standardized classification of adverse events of drugs and treatment used in cancer therapy. The CTCAE system is a product of the US National Cancer Institute (NCI). The first Iteration was prior to 1998. In 1999, the FDA released version 2.0. CTCAE version 4.0 in 2009 with an update to y version 4.03 in 2010. The current version 5.0 was released on November 27, 2017. Many clinical trials, now extending beyond oncology, encode their observations based on the CTCAE system. It uses a range of grades from 1 to 5. Specific conditions and symptom Signs and symptoms are diagnostic indications of an illness, injury, or condition. Signs are objective and externally observable; symptoms are a person's reported subjective experiences. A sign for example may be a higher or lower temperature ...s may have values or descriptive comment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
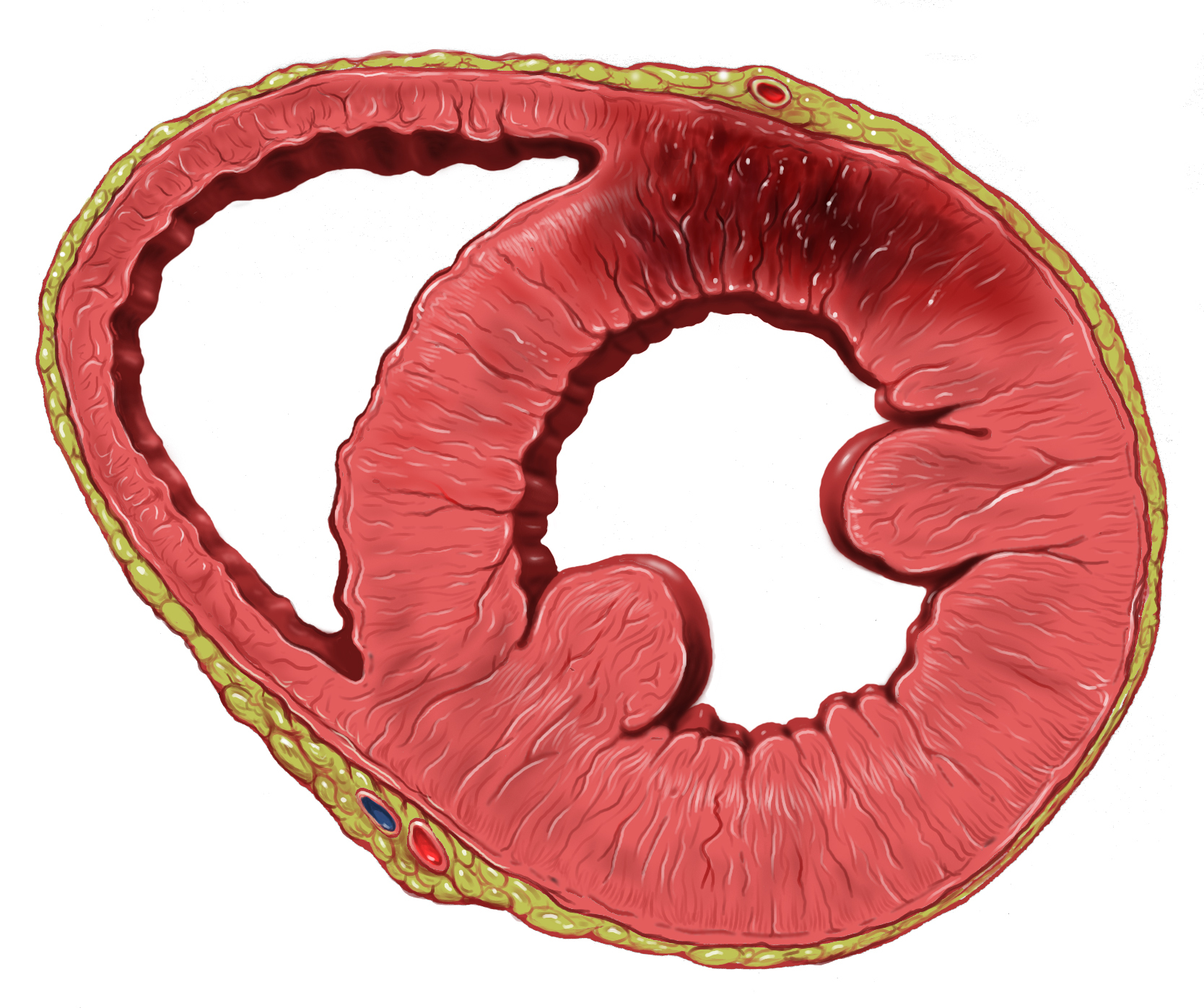
Myocardial Infarction
A myocardial infarction (MI), commonly known as a heart attack, occurs when Ischemia, blood flow decreases or stops in one of the coronary arteries of the heart, causing infarction (tissue death) to the heart muscle. The most common symptom is retrosternal Angina, chest pain or discomfort that classically radiates to the left shoulder, arm, or jaw. The pain may occasionally feel like heartburn. This is the dangerous type of acute coronary syndrome. Other symptoms may include shortness of breath, nausea, presyncope, feeling faint, a diaphoresis, cold sweat, Fatigue, feeling tired, and decreased level of consciousness. About 30% of people have atypical symptoms. Women more often present without chest pain and instead have neck pain, arm pain or feel tired. Among those over 75 years old, about 5% have had an MI with little or no history of symptoms. An MI may cause heart failure, an Cardiac arrhythmia, irregular heartbeat, cardiogenic shock or cardiac arrest. Most MIs occur d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MedDRA
A subscription-based product of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), MedDRA or Medical Dictionary for Regulatory Activities is a clinically validated international medical terminology dictionary-thesaurus used by regulatory authorities and the biopharmaceutical industry during the regulatory process, from pre-marketing (clinical research phase 0 to phase 3) to post-marketing activities (pharmacovigilance or clinical research phase 4), and for safety information data entry, retrieval, evaluation, and presentation. Also, it is the adverse event classification dictionary. The first version of MedDRA was released in 1999 in English and Japanese. MedDRA is now translated into Chinese, Czech, Dutch, French, German, Hungarian, Italian, Korean, Portuguese, Brazilian Portuguese, Russian, and Spanish. In MedDRA version 25.0, Swedish and Latvian translations were also added. In many countries/regions the use of M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triage
In medicine, triage (, ; ) is a process by which care providers such as Health professional, medical professionals and those with first aid knowledge determine the order of priority for providing treatment to injured individuals and/or inform the rationing of limited supplies so that they go to those who can most benefit from it. Triage is usually relied upon when there are more injured individuals than available care providers (known as a mass casualty incident), or when there are more injured individuals than supplies to treat them. The methodologies of triage vary by institution, locality, and country but have the same universal underlying concepts. In most cases, the triage process places the most Major trauma, injured and most able to be helped as the first priority, with the most Terminal illness, terminally injured the last priority (except in the case of reverse triage). Triage systems vary dramatically based on a variety of factors, and can follow specific, measurable me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
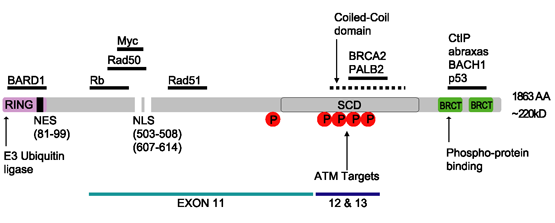
BRCA1
Breast cancer type 1 susceptibility protein is a protein that in humans is encoded by the ''BRCA1'' () gene. Orthologs are common in other vertebrate species, whereas invertebrate genomes may encode a more distantly related gene. ''BRCA1'' is a human tumor suppressor gene (also known as a caretaker gene) and is responsible for repairing DNA. ''BRCA1'' and ''BRCA2'' are unrelated proteins, but both are normally expressed in the cells of breast and other tissues, where they help repair damaged DNA, or destroy cells if DNA cannot be repaired. They are involved in the repair of chromosomal damage with an important role in the error-free repair of DNA double-strand breaks. If ''BRCA1'' or ''BRCA2'' itself is damaged by a BRCA mutation, damaged DNA is not repaired properly, and this increases the risk for breast cancer. ''BRCA1'' and ''BRCA2'' have been described as "breast cancer susceptibility genes" and "breast cancer susceptibility proteins". The predominant allele has a no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Risk Factor
In epidemiology, a risk factor or determinant is a variable associated with an increased risk of disease or infection. Due to a lack of harmonization across disciplines, determinant, in its more widely accepted scientific meaning, is often used as a synonym. The main difference lies in the realm of practice: medicine (clinical practice) versus public health. As an example from clinical practice, low ingestion of dietary sources of vitamin C is a known risk factor for developing scurvy. Specific to public health policy, a determinant is a health risk that is general, abstract, related to inequalities, and difficult for an individual to control. For example, poverty is known to be a determinant of an individual's standard of health. Risk factors may be used to identify high-risk people. Correlation vs causation Risk factors or determinants are correlational and not necessarily causal, because correlation does not prove causation. For example, being young cannot be said to caus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |