|
Diterpenoids
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. Some diterpenes are known to be antimicrobial and anti-inflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranylgeranyl Pyrophosphate
Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and diterpenoids. It is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls. It is also a precursor to geranylgeranylated proteins, which is its primary use in human cells. It is formed from farnesyl pyrophosphate by the addition of an isoprene unit from isopentenyl pyrophosphate. In ''Drosophila'', geranylgeranyl pyrophosphate is synthesised by HMG-CoA encoded by the Columbus gene. Geranylgeranyl pyrophosphate is utilised as a chemoattractant for migrating germ cells that have traversed the midgut epithelia. The attractant signal is produced at the gonadal precursors, directing the germ cells to these sites, where they will differentiate into eggs and spermatozoa (sperm). Related compounds * Farnesyl pyrophosphate * Geranylgeraniol Geranylgeraniol is a diterpenoid alcohol. It is a colorless waxy solid. It is an important intermediate in the biosynthesis of other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stemarene
Stemarene is a diterpene hydrocarbon can be produced biosynthetically through enzyme An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ... extracts from rice. References Diterpenes Cyclopentanes {{alkanederivative-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinol
Retinol, also called vitamin A1, is a fat-soluble vitamin in the vitamin A family that is found in food and used as a dietary supplement. Retinol or other forms of vitamin A are needed for vision, cellular development, maintenance of skin and mucous membranes, immune function and reproductive development. Dietary sources include fish, dairy products, and meat. As a supplement it is used to treat and prevent vitamin A deficiency, especially that which results in xerophthalmia. It is taken by mouth or by intramuscular injection, injection into a muscle. As an ingredient in skin-care products, it is used to reduce wrinkles and other effects of skin aging. Retinol at normal doses is well tolerated. High doses may cause hepatomegaly, enlargement of the liver, dry skin, and hypervitaminosis A. High doses during pregnancy may harm the fetus. The body converts retinol to retinal and retinoic acid, through which it acts. Retinol was discovered in 1909, isolated in 1931, and first m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phylloquinone
Phytomenadione, also known as vitamin K1, phylloquinone, or phytonadione, is a vitamin found in food and used as a dietary supplement. It is on the World Health Organization's List of Essential Medicines. It is used to treat certain bleeding disorders, including warfarin overdose, vitamin K deficiency, and obstructive jaundice. Use is typically recommended by mouth, intramuscular injection or injection under the skin. When given by injection benefits are seen within two hours. It is also recommended for preventing and treating vitamin K deficiency bleeding in infants. Many countries in the world choose intramuscular injections in newborn to keep them safe from vitamin K deficiency bleeding. It is considered a safe treatment and saves many children from death and severe neurologic deficit every year. Side effects when given by injection may include pain at the site of injection. Severe allergic reactions may occur when it is injected into a vein or muscle, but this has m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
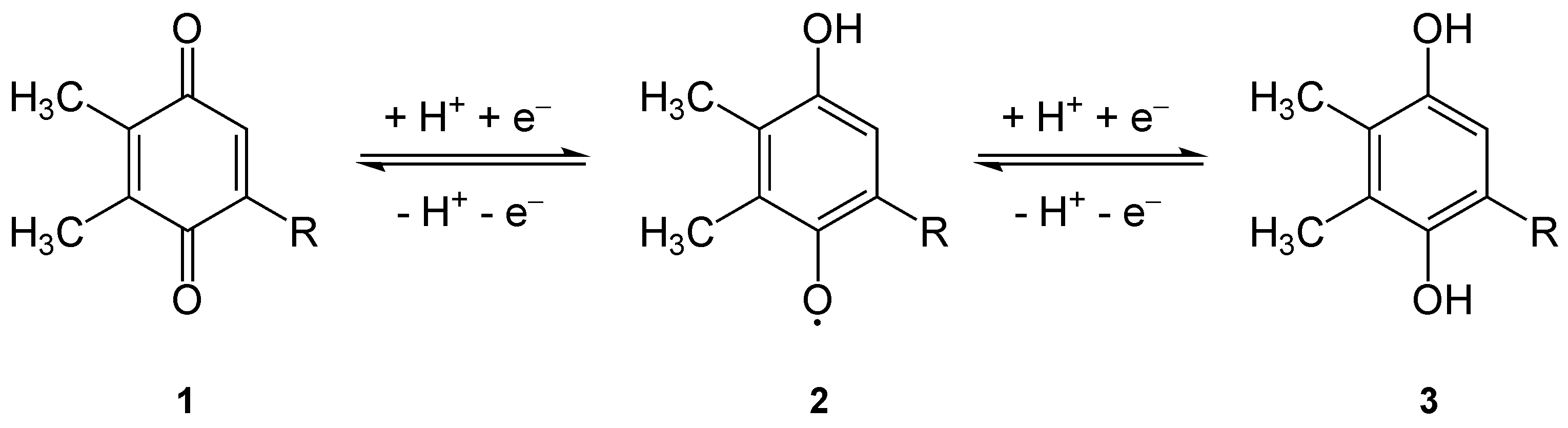
Plastoquinone
Plastoquinone (PQ) is a terpenoid-quinone ( meroterpenoid) molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. The most common form of plastoquinone, known as PQ-A or PQ-9, is a 2,3-dimethyl-1,4- benzoquinone molecule with a side chain of nine isoprenyl units. There are other forms of plastoquinone, such as ones with shorter side chains like PQ-3 (which has 3 isoprenyl side units instead of 9) as well as analogs such as PQ-B, PQ-C, and PQ-D, which differ in their side chains. The benzoquinone and isoprenyl units are both nonpolar, anchoring the molecule within the inner section of a lipid bilayer, where the hydrophobic tails are usually found. Plastoquinones are very structurally similar to ubiquinone, or coenzyme Q10, differing by the length of the isoprenyl side chain, replacement of the methoxy groups with methyl groups, and removal of the methyl group in the 2 position on the quinone. Like ubiquinone, it can come in severa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
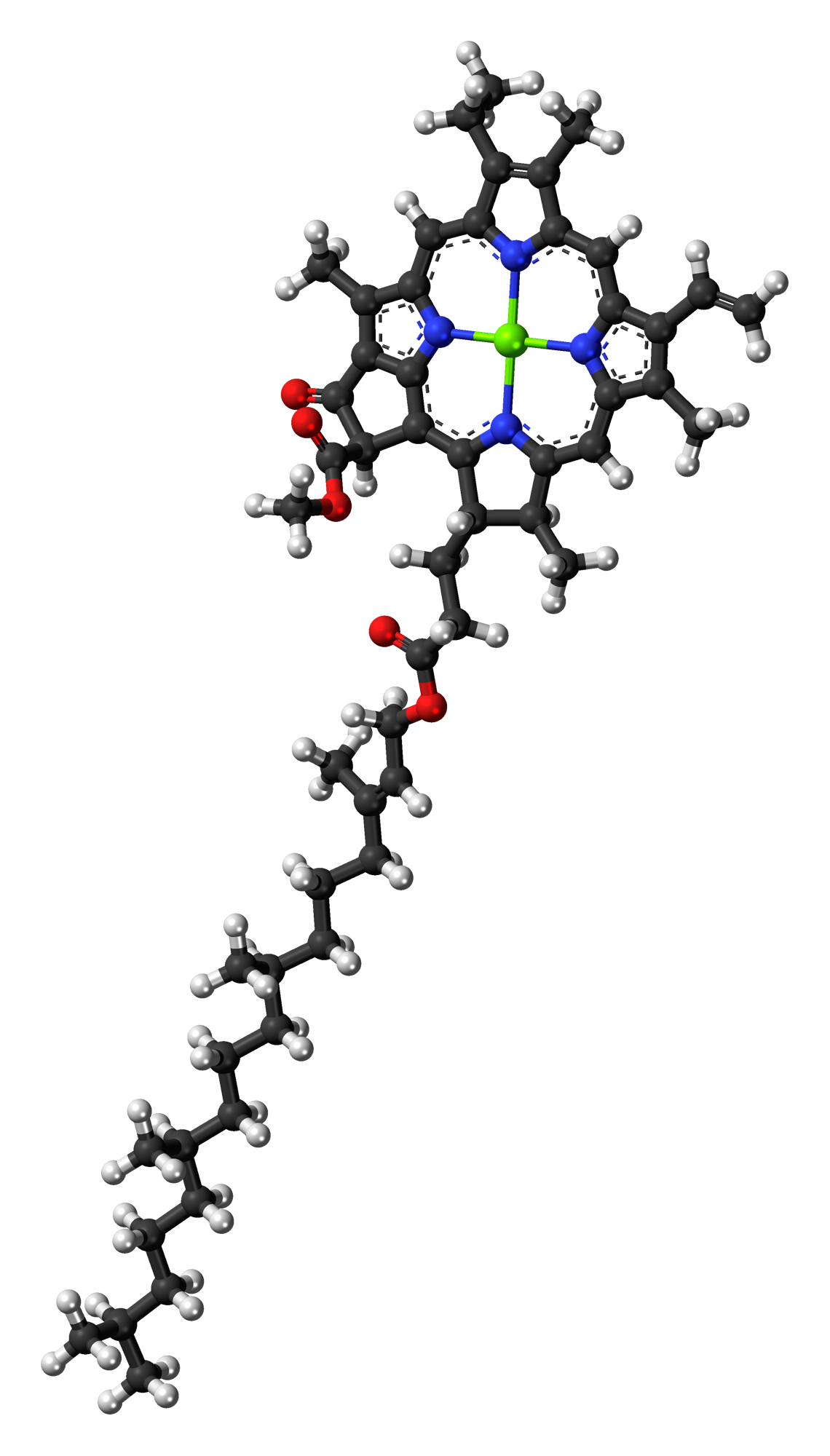
Ubiquinone
Coenzyme Q10 (CoQ10 ), also known as ubiquinone, is a naturally occurring Cofactor (biochemistry), biochemical cofactor (coenzyme) and an antioxidant produced by the human body. It can also be obtained from dietary sources, such as meat, fish, seed oils, vegetables, and dietary supplements. CoQ10 is found in many organisms, including animals and bacteria. CoQ10 plays a role in mitochondrial oxidative phosphorylation, aiding in the production of adenosine triphosphate (ATP), which is involved in energy transfer within cells. The structure of CoQ10 consists of a benzoquinone moiety and an isoprenoid side chain, with the "10" referring to the number of Isoprene, isoprenyl chemical subunits in its tail. Although a ubiquitous molecule in human tissues, CoQ10 is not a dietary nutrient and does not have a Dietary Reference Intake, recommended intake level, and its use as a supplement is not approved drug, approved in the United States for any health or anti-disease effect. Biologica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll A
} Chlorophyll ''a'' is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light, and it is a poor absorber of green and near-green portions of the spectrum. Chlorophyll does not reflect light but chlorophyll-containing tissues appear green because green light is diffusively reflected by structures like cell walls. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain. Chlorophyll ''a'' also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located. Distribution of chlorophyll ''a'' Chlorophyll ''a'' is essential for most photosynthetic organisms to release chemical energy but is not the only pigment that can be used for photosynthesis. All oxygenic photosynthetic organisms use chloroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
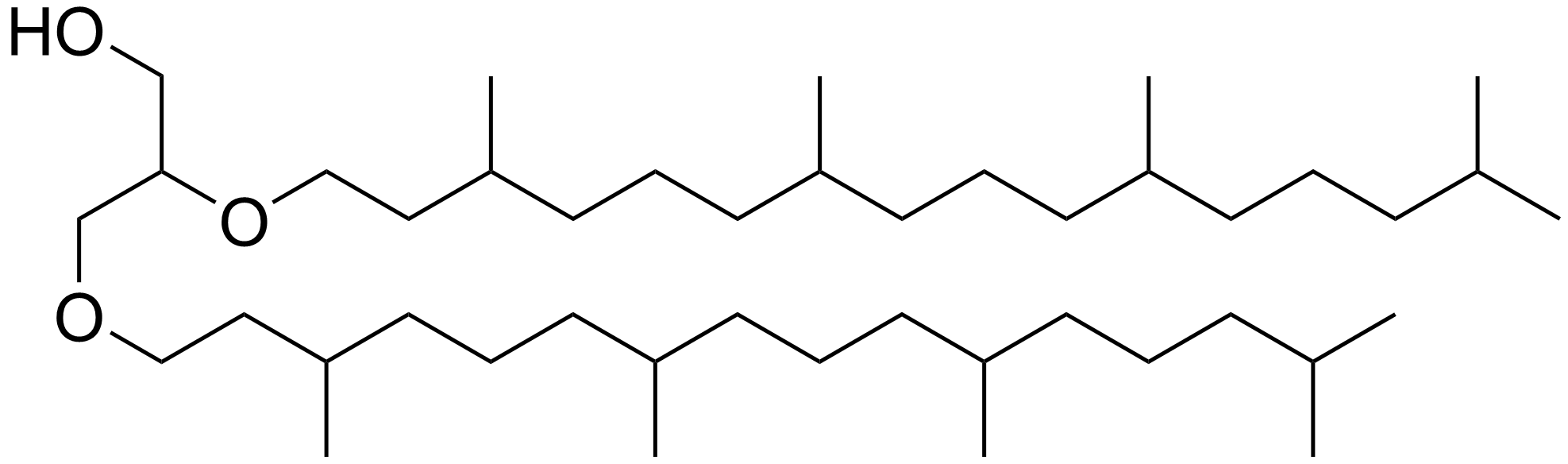
Phytyl
Phytane is the isoprenoid alkane formed when phytol, a chemical substituent of chlorophyll, loses its hydroxyl group. When phytol loses one carbon atom, it yields pristane. Other sources of phytane and pristane have also been proposed than phytol. Pristane and phytane are common constituents in petroleum and have been used as proxies for depositional redox conditions, as well as for correlating oil and its source rock (i.e. elucidating where oil formed). In environmental studies, pristane and phytane are target compounds for investigating oil spills. Chemistry Phytane is a non-polar organic compound that is a clear and colorless liquid at room temperature. It is a head-to-tail linked regular isoprenoid with chemical formula C20H42. Phytane has many structural isomers. Among them, crocetane is a tail-to-tail linked isoprenoid and often co-elutes with phytane during gas chromatography (GC) due to its structural similarity. Phytane also has many stereoisomers because of i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tocopherol
Tocopherols (; TCP) are a class of organic compounds comprising various methylated phenols, many of which have vitamin E activity. Because the vitamin activity was first identified in 1936 from a dietary fertility factor in rats, it was named ''tocopherol'', from Greek τόκος ''tókos'' 'birth' and φέρειν ''phérein'' 'to bear or carry', that is 'to carry a pregnancy', with the ending ''-ol'' signifying its status as a chemical alcohol. α-Tocopherol is the main source found in supplements and in the European diet, where the main dietary sources are olive and sunflower oils, while γ-tocopherol is the most common form in the American diet due to a higher intake of soybean and corn oil. Forms Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain that allows for penetration into biological membranes. B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytane
Phytane is the Diterpenoid, isoprenoid alkane formed when phytol, a chemical substituent of chlorophyll, loses its Hydroxy group, hydroxyl group. When phytol loses one carbon atom, it yields pristane. Other sources of phytane and pristane have also been proposed than phytol. Pristane and phytane are common constituents in petroleum and have been used as Proxy (climate), proxies for Deposition (geology), depositional redox conditions, as well as for correlating oil and its source rock (i.e. elucidating where oil formed). In environmental studies, pristane and phytane are target compounds for investigating oil spills. Chemistry Phytane is a Chemical polarity, non-polar organic compound that is a clear and colorless liquid at room temperature. It is a wikibooks:Structural Biochemistry/Lipids/Isoprenoids#Structural Features and Some Isoprenoid Compounds, head-to-tail linked regular Terpenoid, isoprenoid with chemical formula C20H42. Phytane has List of straight-chain alkanes, many S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteria's informal common name, blue-green algae. Cyanobacteria are probably the most numerous taxon to have ever existed on Earth and the first organisms known to have produced oxygen, having appeared in the middle Archean eon and apparently originated in a freshwater or terrestrial environment. Their photopigments can absorb the red- and blue-spectrum frequencies of sunlight (thus reflecting a greenish color) to split water molecules into hydrogen ions and oxygen. The hydrogen ions are used to react with carbon dioxide to produce complex organic compounds such as carbohydrates (a process known as carbon fixation), and the oxygen is released as a byproduct. By continuously producing and releasing oxygen over billions of years, cyanobacte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. In mammals, these enzymes oxidize steroids, fatty acids, xenobiotics, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted. P450s are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived from the spectrophotometry, spectrophotometric peak at the wavelength of the absorption spectroscopy, absorption maximum of the enzyme (450 nanometre, nm) when it is in the redox, reduced state and complexed with carbon monoxide. Most P450s require a protein partner to deliver one or more electrons to reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |