|
Disposable Batteries
A primary battery or primary cell is a battery (a galvanic cell) that is designed to be used once and discarded, and it is not rechargeable unlike a secondary cell (rechargeable battery). In general, the electrochemical reaction occurring in the cell is not reversible, rendering the cell unrechargeable. As a primary cell is used, chemical reactions in the battery use up the chemicals that generate the power; when they are gone, the battery stops producing electricity. In contrast, in a secondary cell, the reaction can be reversed by running a current into the cell with a battery charger to recharge it, regenerating the chemical reactants. Primary cells are made in a range of standard sizes to power small household appliances such as flashlights and portable radios. Primary batteries make up about 90% of the $50 billion battery market, but secondary batteries have been gaining market share. About 15 billion primary batteries are thrown away worldwide every year, virtually all endi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Batteries Comparison 4,5 D C AA AAA AAAA A23 9V CR2032 LR44 Matchstick-1
Battery or batterie most often refers to: * Electric battery, a device that provides electrical power * Battery (crime), a crime involving unlawful physical contact Battery may also refer to: Energy source *Battery indicator, a device which gauges the state of charge for electronics *Energy storage, including batteries that are not electrochemical *List of battery types Law * Battery (tort), a civil wrong in common law of intentional harmful or offensive contact Military and naval uses * Artillery battery, an organized group of artillery pieces ** Main battery, the primary weapons of a warship ** Secondary battery (artillery), the smaller guns on a warship * Battery, a position of a cartridge in a firearm action Arts and entertainment Music * Battery (electro-industrial band) * Battery (hardcore punk band) * Battery (song), "Battery" (song), a song by Metallica from the 1986 album ''Master of Puppets'' * Drums, which have historically been grouped into ensembles called a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rechargeable Batteries
A rechargeable battery, storage battery, or secondary cell (formally a type of energy accumulator), is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer (Li-ion polymer). Rechargeable batteries typically initially cost more th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
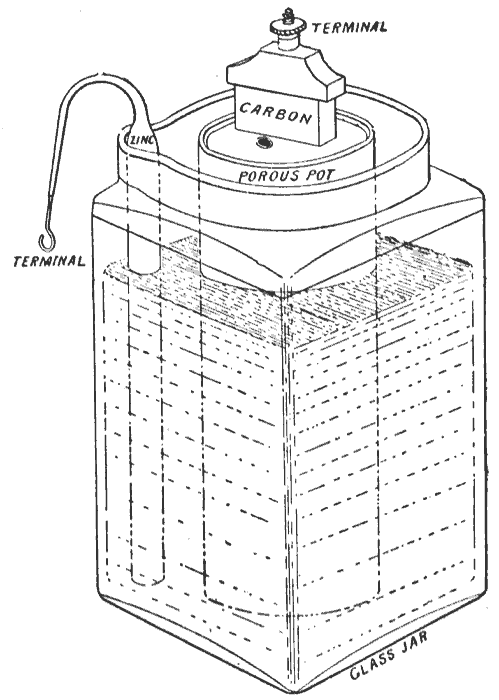
Grove Cell
The Grove cell was an early electric primary cell named after its inventor, Welsh physical scientist William Robert Grove, and consisted of a zinc anode in dilute sulfuric acid and a platinum cathode in concentrated nitric acid, the two separated by a porous ceramic pot. Cell details The Grove cell voltage is about 1.9 volts and arises from the following reaction: : Zn + H2SO4 + 2 HNO3 ZnSO4 + 2 H2O + 2 NO2↑ Use The Grove cell was the favored power source of the early American telegraph system in the period 1840 – 1860 because it offered a high current output and higher voltage than the earlier Daniell cell (at 1.9 volts and 1.1 volts, respectively). Disadvantages By the time of the American Civil War, as telegraph traffic increased, the Grove cell's tendency to discharge poisonous nitrogen dioxide (NO2) fumes proved increasingly hazardous to health, and as telegraphs became more complex, the need for constant voltage became critical. The Grove cell was limited in this r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bunsen Cell
The Bunsen cell is a zinc-carbon primary cell (colloquially called a "battery") composed of a zinc anode in dilute sulfuric acid separated by a porous pot from a carbon cathode in Nitric acid, nitric or chromic acid. Cell details The Bunsen cell generates about 1.9 volts which arises from the following reaction: : Zn + H2SO4 + 2 HNO3 ZnSO4 + 2 H2O + 2 NO2(g) According to the reaction above, when 1 mole (unit), mole (or part) each of zinc and sulfuric acid react with 2 moles (or parts) of nitric acid, the resultant products formed are, 1 mole (or part) of zinc sulfate and 2 moles (or parts) each of water and nitrogen dioxide (gaseous, in the form of bubbles). The cell is named after its inventor, German chemist Robert Wilhelm Bunsen, who improved upon the Grove cell by replacing William Robert Grove, Grove's expensive platinum cathode with carbon in the form of pulverized coal and coke (fuel), coke. Like Grove's battery, Bunsen's emitted noxious fumes of nitrogen dioxide. Bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leclanché Cell
The Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché in 1866. The battery contained a conducting solution (electrolyte) of ammonium chloride, a cathode (positive terminal) of carbon, a depolarizer of manganese dioxide (oxidizer), and an anode (negative terminal) of zinc (reductant). The chemistry of this cell was later successfully adapted to manufacture a dry cell. History In 1866, Georges Leclanché invented a battery that consisted of a zinc anode and a manganese dioxide cathode wrapped in a porous material, dipped in a jar of ammonium chloride solution. The manganese dioxide cathode had a little carbon mixed into it as well, which improved conductivity and absorption. It provided a voltage of 1.4 volts. This cell achieved very quick success in telegraphy, signalling and electric bell work. The dry cell form was used to power early telephones—usually from an adjacent wooden box affixed to the wall—before telephones could dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery, although it is also used for other battery chemistries such as aqueous zinc-ion batteries.. is also used as a pigment and as a precursor to other manganese compounds, such as . It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. has an α- polymorph that can incorporate a variety of atoms (as well as water molecules) in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in as a possible cathode for lithium-ion batteries. Structure Several polymorphs of are claimed, as well as a hydrated form. Like many other dioxides, crystallizes in the rutile crystal structure (t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current describes the direction in which positive charges move. Electrons, which are the carriers of current in most electrical systems, have a negative electrical charge, so the movement of electrons is ''opposite'' to that of the conventional current flow: this means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Munitions
Ammunition, also known as ammo, is the material fired, scattered, dropped, or detonated from any weapon or weapon system. The term includes both expendable weapons (e.g., bombs, missiles, grenades, land mines), and the component parts of other weapons that create the effect on a target (e.g., bullets and warheads). The purpose of ammunition is to project a force against a selected target to have an effect (usually, but not always, lethal). An example of ammunition is the firearm cartridge, which includes all components required to deliver the weapon effect in a single package. Until the 20th century, black powder was the most common propellant used but has now been replaced in nearly all cases by modern compounds. Ammunition comes in a great range of sizes and types and is often designed to work only in specific weapons systems. However, there are internationally recognized standards for certain ammunition types (e.g., 5.56×45mm NATO) that enable their use across diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |