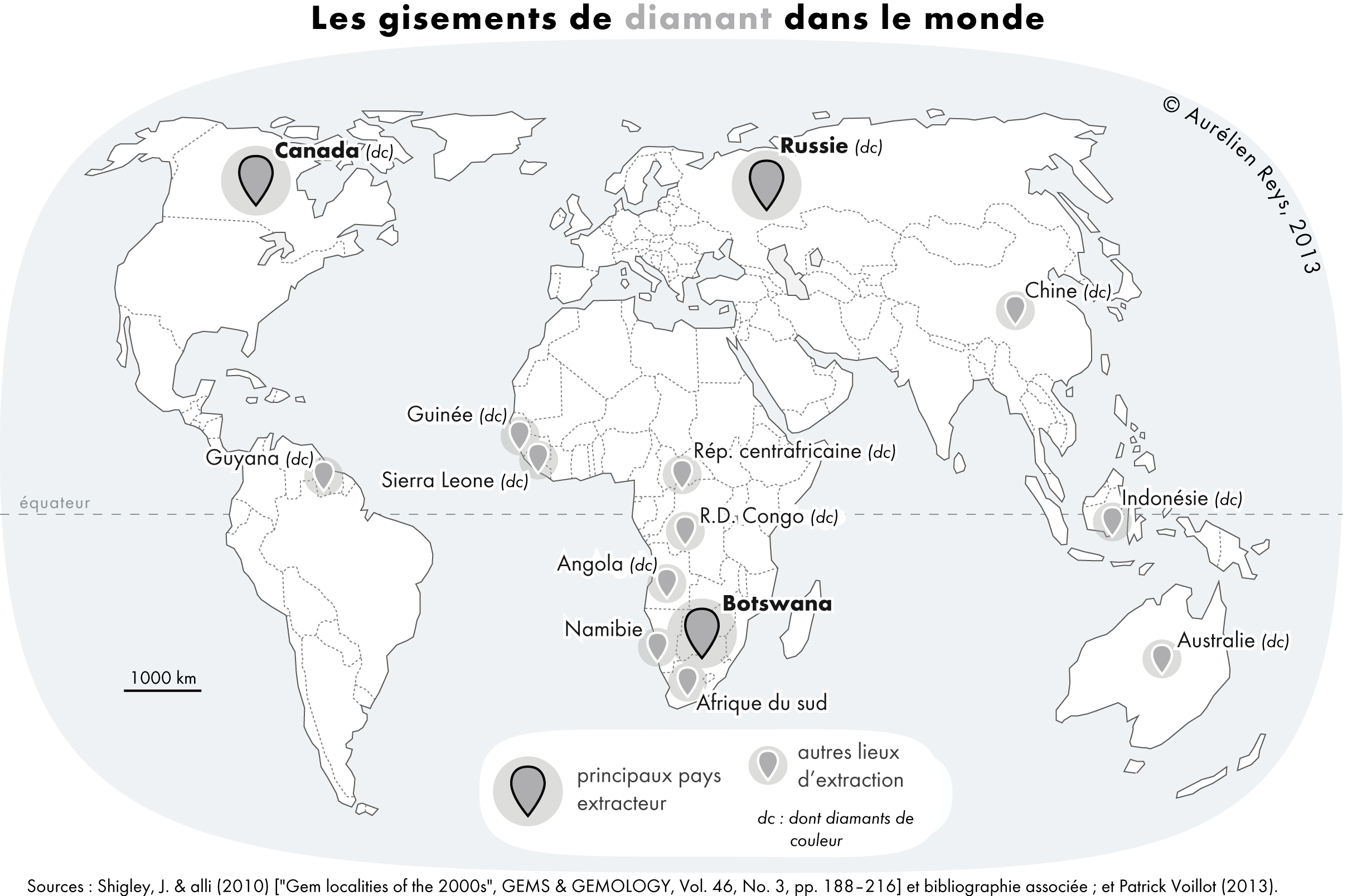
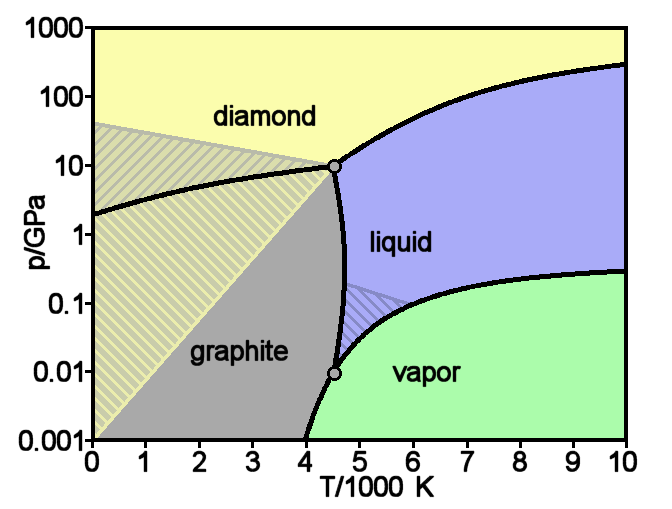
|
Diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of carbon at Standard temperature and pressure, room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest Scratch hardness, hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of lattice defect, defects or impurities (about one per million of lattice atoms) can color ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Diamond
A synthetic diamond or laboratory-grown diamond (LGD), also called a lab-grown, laboratory-created, man-made, artisan-created, artificial, or cultured diamond, is a diamond that is produced in a controlled technological process, in contrast to a naturally-formed diamond, which is created through geological processes and obtained by mining. Unlike diamond simulants (imitations of diamond made of superficially similar non-diamond materials), synthetic diamonds are composed of the same material as naturally formed diamonds—pure carbon crystallized in an isotropic 3D form—and have identical chemical and physical properties. The maximal size of synthetic diamonds has increased dramatically in the 21st century. Before 2010, most synthetic diamonds were smaller than half a carat. Improvements in technology, plus the availability of larger diamond substrates, have led to synthetic diamonds up to 125 carats in 2025.=https://www.gia.edu/gia-news-research/gems-gemology-summary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Carbon
Carbon is capable of forming many allotropy, allotropes (structurally different forms of the same element) due to its Valence (chemistry), valency (Tetravalence, tetravalent). Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include carbon nano tube, nanotubes, Carbon nanobud, nanobuds and Graphene nanoribbon, nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA). Atomic and diatomic carbon Under certain conditions, carbon can be found in its atomic form. It can be formed by vaporizing graphite, by passing large electric currents to form a carbon arc under very low pressure. It is extrem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films. In typical CVD, the wafer (electronics), wafer (substrate) is exposed to one or more Volatility (chemistry), volatile wikt:precursor, precursors, which chemical reaction, react and/or chemical decomposition, decompose on the substrate surface to produce the desired deposit. Frequently, volatile by-products are also produced, which are removed by gas flow through the reaction chamber. Microfabrication processes widely use CVD to deposit materials in various forms, including: Single crystal, monocrystalline, polycrystalline, amorphous, and Epitaxy, epitaxial. These materials include: silicon (Silicon dioxide, dioxide, silicon carbide, carbide, silicon nitride, nitride, silicon oxynitride, oxynitride), carbon (carbon (fiber), fiber, carbon nanofibers, nanofibers, carbon nanot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kimberlite
Kimberlite is an igneous rock and a rare variant of peridotite. It is most commonly known as the main host matrix for diamonds. It is named after the town of Kimberley, Northern Cape, Kimberley in South Africa, where the discovery of an 83.5-Carat (mass), carat (16.70 g) diamond called the Star of South Africa (diamond), Star of South Africa in 1869 spawned a diamond rush and led to the excavation of the open-pit mine called the Big Hole. Previously, the term kimberlite has been applied to olivine lamproites as Kimberlite II, however this has been in error. Kimberlite occurs in the Earth's Crust (geology), crust in vertical structures known as Volcanic pipe, kimberlite pipes, as well as igneous Dike (geology), dykes and can also occur as horizontal Sill (geology), sills. Kimberlite pipes are the most important source of mined diamonds today. The consensus on kimberlites is that they are formed deep within Earth's mantle. Formation occurs at depths between 150 and 450 kilometres ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond Cubic
In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, including α-tin, the semiconductors silicon and germanium, and silicon–germanium alloys in any proportion. There are also crystals, such as the high-temperature form of cristobalite, which have a similar structure, with one kind of atom (such as silicon in cristobalite) at the positions of carbon atoms in diamond but with another kind of atom (such as oxygen) halfway between those (see :Minerals in space group 227). Although often called the diamond lattice, this structure is not a lattice in the technical sense of this word used in mathematics. Crystallographic structure Diamond's cubic structure is in the Fdm space group (space group 227), which follows the face-centered cubic Bravais lattice. The lattice describes the repeat pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rough Diamond
A rough diamond is a diamond that has not been cut or processed. They come in a variety of naturally occurring shapes, including octahedral (eight-sided bipyramid), cubic, and triangular (most commonly macles). A raw diamond or rough diamond can also be a type of diamond that is not fully developed or can have less brilliance. Extreme heat and pressure beneath the ground make the carbon atoms fuse in a specific structure. See also *Bort * Carbonado * Diamond clarity * Diamond type *List of diamonds Diamond (gemstone), Diamonds occur naturally and vary in size, color, and quality, so the largest of a particular color may not be large in absolute terms, but may still be considered very desirable. Diamonds may also have high valuations in sal ... References External linksRaw Diamondat Gemone Diamond *Loose Diamonds diamond engagement ringsat Earthshine Jewels Diamond {{mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lamproite
Lamproite is an ultrapotassic mantle-derived volcanic or subvolcanic rock. It has low CaO, Al2O3, Na2O, high K2O/Al2O3, a relatively high MgO content and extreme enrichment in incompatible elements. Lamproites are geographically widespread yet are volumetrically insignificant. Unlike kimberlites, which are found exclusively in Archaean cratons, lamproites are found in terrains of varying age, ranging from Archaean in Western Australia, to Palaeozoic and Mesozoic in southern Spain. They also vary widely in age, from Proterozoic to Pleistocene, the youngest known example from Gaussberg in Antarctica being 56,000 ± 5,000 years old. Lamproite volcanology is varied, with both diatreme styles and cinder cone or cone edifices known. Petrology Lamproites form from partially melted mantle at depths exceeding 150 km. The molten material is forced to the surface in volcanic pipes, bringing with it xenoliths and diamonds from the harzburgitic peridotite or eclogite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials such as mineral wool or Styrofoam. Metals have this high thermal conductivity due to free electrons facilitating heat transfer. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovas and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is found in smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scratch Hardness
Scratch hardness refers to the hardness of a material in terms of resistance to scratches and abrasion by a harder material forcefully drawn over its surface. Scratch hardness test or scratch test refers to any of a number of methods of measuring scratch hardness. Resistance to abrasion is less affected by surface variations than indentation methods. Scratch hardness is measured with a sclerometer. Attempting to scratch a surface to test a material is a very old technique. The first ''scientific'' attempt to quantify materials by scratch tests was by mineralogist Friedrich Mohs in 1812 (see Mohs scale)."Mohs hardness" in ''Encyclopædia Britannica Online'' The Mohs scale is based on relative scratch hardness of different materials; with |