|
C–H···O Interaction
In chemistry, a C–H···O interaction is occasionally described as a special type of weak hydrogen bond. These interactions frequently occur in the structures of important biomolecules like amino acids, proteins, sugars, DNA and RNA. History The C–H···O interaction was discovered in 1937 by Samuel Glasstone. Glasstone studied properties of mixtures of acetone with different halogenated derivatives of hydrocarbons and realized that dipole moments of these mixtures differ from dipole moments of pure substances. He explained this by establishing the concept of C–H···O interactions. The first crystallographic analysis of C-H ⋯O hydrogen bonds were published by June Sutor in 1962. Properties Similar to hydrogen bonds, a C–H···O interaction involves interactions of dipoles and therefore has directionality. The directionality of a C–H···O interaction is usually defined by the angle ''α'' between the С, Н and О atoms, and the distance ''d'' between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
June Sutor
Dorothy June Sutor (6 June 1929 – 27 May 1990) was a New Zealand-born crystallographer who spent most of her research career in England. She was one of the first scientists to establish that hydrogen bonds could form to hydrogen atoms bonded to carbon atoms. She later worked in the laboratory of Kathleen Lonsdale on the characterisation and prevention of urinary calculi. Early life and education Sutor was born in New Zealand, in the Auckland suburb of Parnell, on 6 June 1929, the daughter of Victor Edward Sutor, a coach builder, and Cecilia Maud Sutor (née Craner). She was educated at St Cuthbert's College, and went on to study chemistry at Auckland University College. She graduated Master of Science with first-class honours in 1952 and, supervised by Frederick Llewellyn, she graduated with her first PhD in 1954. She published her first single-author ''Acta Crystallographica'' paper, ''The unit cell and space group of ethyl nitrolic acid'', whilst a student. In 1954, S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
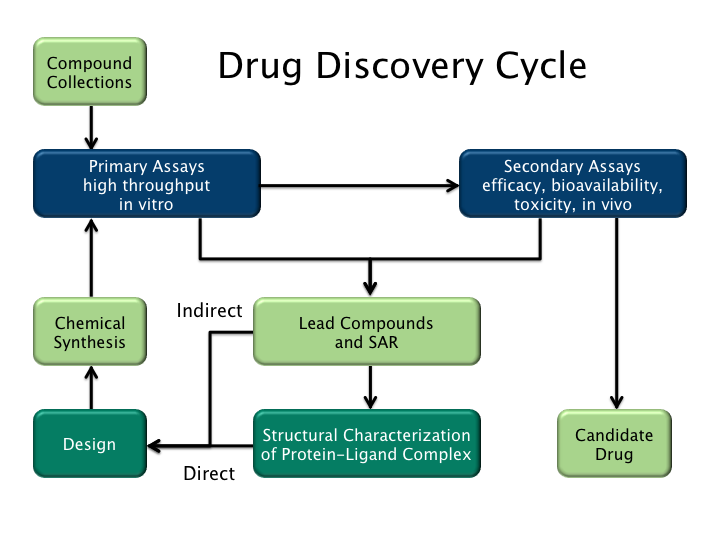
Drug Design
Drug design, often referred to as rational drug design or simply rational design, is the invention, inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic compound, organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic effect, therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and electric charge, charge to the biomolecular target with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on molecular modelling, computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the biomolecular target is known as structure-based drug design. In addition to small molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycyclic Aromatic Hydrocarbon
A Polycyclic aromatic hydrocarbon (PAH) is any member of a class of organic compounds that is composed of multiple fused aromatic rings. Most are produced by the incomplete combustion of organic matter— by engine exhaust fumes, tobacco, incinerators, in roasted meats and cereals, or when biomass burns at lower temperatures as in forest fires. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are also found in fossil fuel deposits such as coal and in petroleum. Exposure to PAHs can lead to different types of cancer, to fetal development complications, and to cardiovascular issues. Polycyclic aromatic hydrocarbons are discussed as possible starting materials for abiotic syntheses of materials required by the earliest forms of life. Nomenclature and structure The terms polyaromatic hydrocarbon, or polynuclear aromatic hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
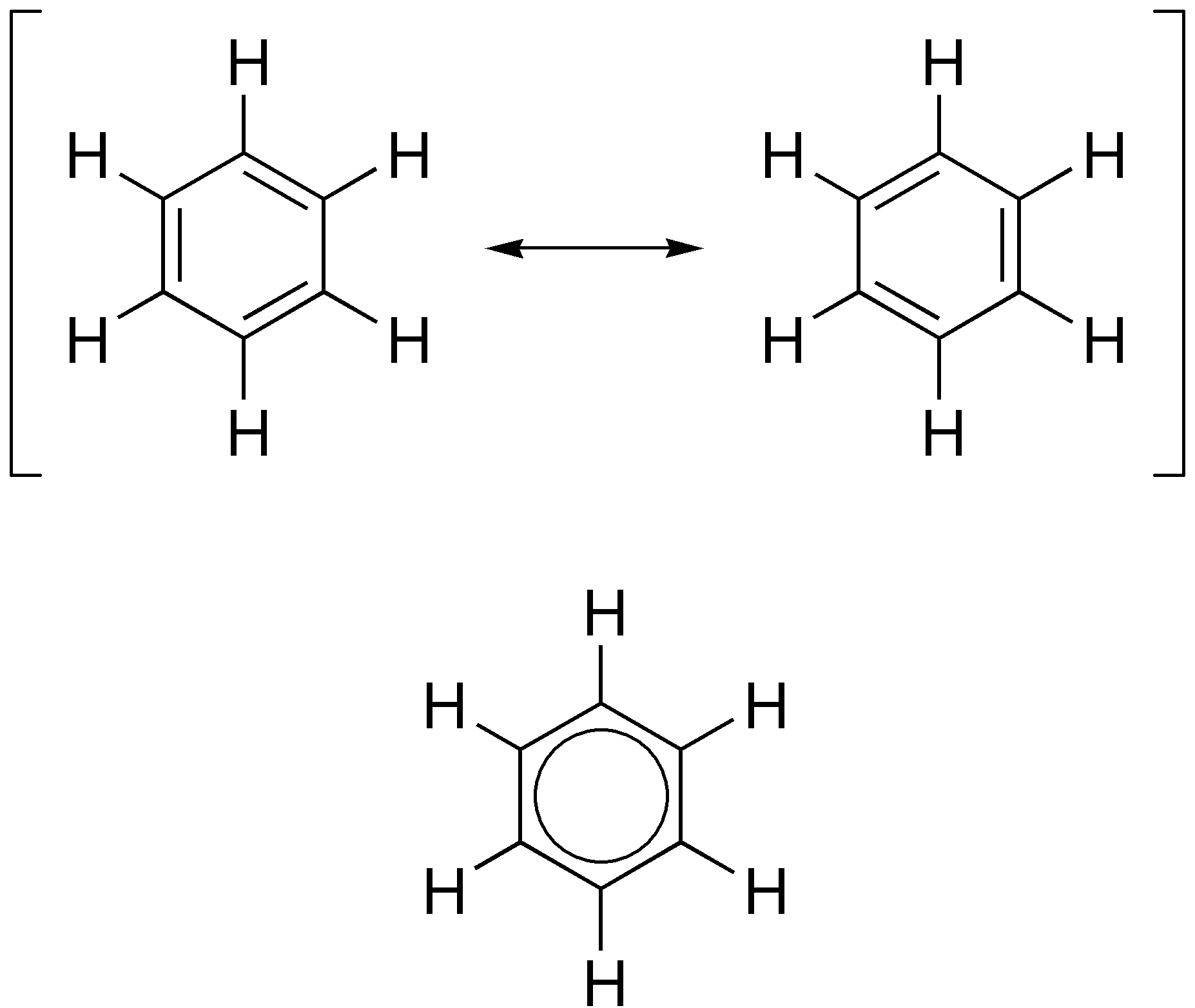
Aromatic Ring
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double- bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). Each bond may be seen as a hybrid of a single bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfaction, olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of Resonance (chemistry), resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-covalent bond, bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Friedrich August Kekulé ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angstrom
The angstrom (; ) is a unit of length equal to m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). It was originally spelled with Swedish letters, as Ångström and later as ångström (). The latter spelling is still listed in some dictionaries, but is now rare in English texts. Some popular US dictionaries list only the spelling ''angstrom''. The unit's symbol is Å, which is a letter of the Swedish alphabet, regardless of how the unit is spelled. However, "A" or "A.U." may be used in less formal contexts or typographically limited media. The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals, wavelengths of electromagnetic radiation, and dimensions of integrated circuit part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Directionality (molecular Biology)
Directionality, in molecular biology and biochemistry, is the end-to-end chemical orientation of a single strand of nucleic acid. In a single strand of DNA or RNA, the chemical convention of naming carbon atoms in the nucleotide Pentose, pentose-sugar-ring means that there will be a 5′ end (usually pronounced "five-prime end"), which frequently contains a phosphate group attached to the 5′ carbon of the ribose ring, and a 3′ end (usually pronounced "three-prime end"), which typically is unmodified from the ribose -OH substituent. In a DNA double helix, the strands run in opposite directions to permit base pairing between them, which is essential for replication or Transcription (biology), transcription of the encoded information. Nucleic acids can only be synthesized in vivo in the 5′-to-3′ direction, as the polymerases that assemble various types of new strands generally rely on the energy produced by breaking nucleoside triphosphate bonds to attach new nucleo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-covalent Interactions
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/ mol (1000–5000 calories per 6.02 molecules). Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects. Non-covalent interactions are critical in maintaining the three-dimensional structure of large molecules, such as proteins and nucleic acids. They are also involved in many biological processes in which large molecules bind specifically but transiently to one another (see the properties section of the DNA page). These interactions also heavily influence drug design, crystallinity and design of materials, particularly for self-asse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bonds
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, covalently bonded to a more electronegative donor atom or group (Dn), interacts with another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Unlike simple dipole–dipole interactions, hydrogen bonding arises from charge transfer (nB → σ*AH), orbital interactions, and quantum mechanical delocalization, making it a resonance-assisted interaction rather than a mere electrostatic attraction. The general notation for hydrogen bonding is Dn−H···Ac, where the solid line represents a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are nitrogen (N), oxygen (O), and fluorine (F), due to their high electronegativity and ability to engage in stronger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently bonded to a more Electronegativity, electronegative donor atom or group (Dn), interacts with another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Unlike simple Dipole–dipole attraction, dipole–dipole interactions, hydrogen bonding arises from charge transfer (nB → σ*AH), Atomic orbital, orbital interactions, and quantum mechanical Delocalized electron, delocalization, making it a resonance-assisted interaction rather than a mere electrostatic attraction. The general notation for hydrogen bonding is Dn−H···Ac, where the solid line represents a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are nitrogen (N), oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |