|
Crystallization
Crystallization is a process that leads to solids with highly organized Atom, atoms or Molecule, molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regular organization. Crystallization can occur by various routes including Precipitation (chemistry), precipitation from solution, freezing of a liquid, or Deposition (phase transition), deposition from a gas. Attributes of the resulting crystal can depend largely on factors such as temperature, air pressure, cooling rate, or Solution (chemistry), solute concentration. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a Supercooling, supercooled liquid or a supersaturation, supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallizer
Crystallization is a process that leads to solids with highly organized atoms or molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regular organization. Crystallization can occur by various routes including precipitation from solution, freezing of a liquid, or deposition from a gas. Attributes of the resulting crystal can depend largely on factors such as temperature, air pressure, cooling rate, or solute concentration. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. Crystallizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
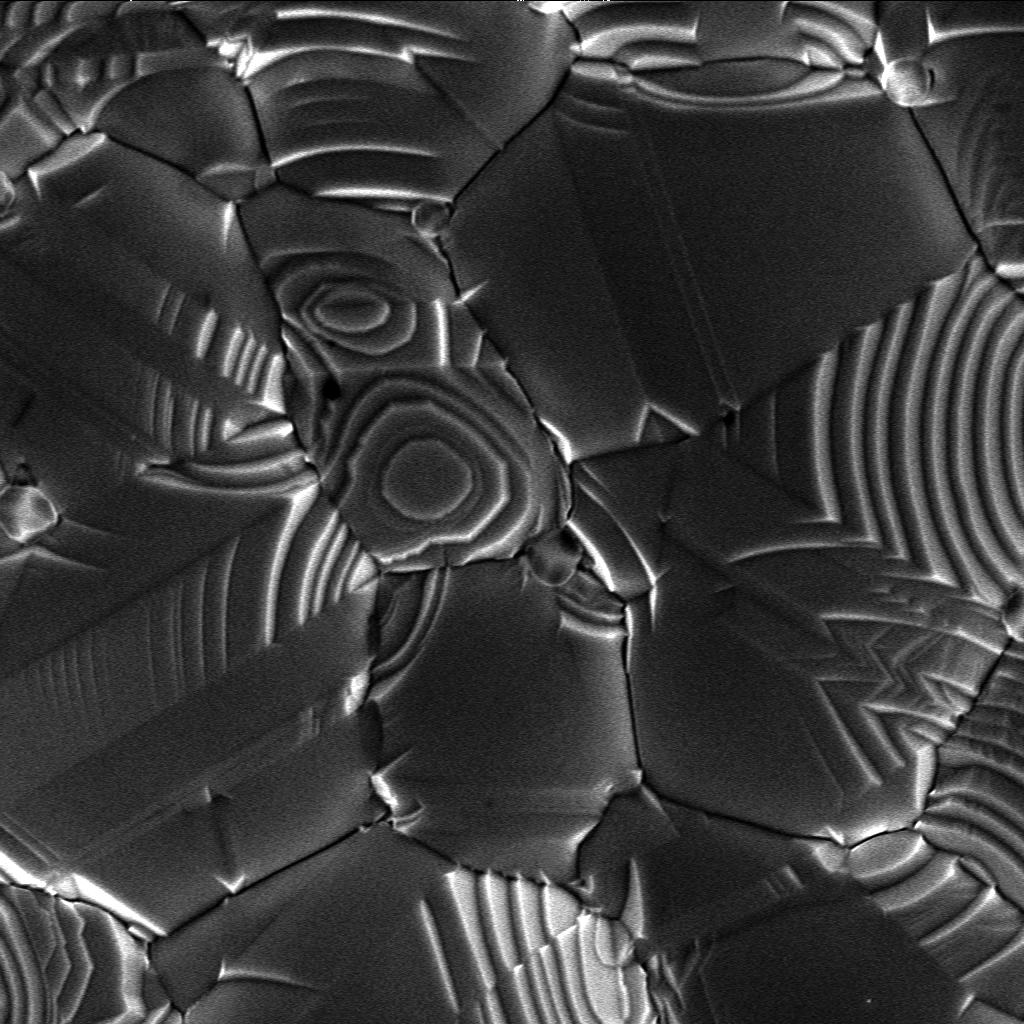
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and " rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supersaturation
In physical chemistry, supersaturation occurs with a solution (chemistry), solution when the concentration of a solute exceeds the concentration specified by the value of solubility at Solubility equilibrium, equilibrium. Most commonly the term is applied to a solution of a solid in a liquid, but it can also be applied to liquids and gases dissolved in a liquid. A supersaturated solution is in a metastable state; it may return to equilibrium by separation process, separation of the excess of solute from the solution, by dilution of the solution by adding solvent, or by increasing the solubility of the solute in the solvent. History Early studies of the phenomenon were conducted with sodium sulfate, also known as Glauber's Salt because, unusually, the solubility of this salt in water may decrease with increasing temperature. Early studies have been summarised by Tomlinson. It was shown that the crystallization of a supersaturated solution does not simply come from its agitation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Growth
Crystal growth is a major stage of a crystallization, crystallization process, and consists of the addition of new atoms, ions, or polymer strings into the characteristic arrangement of the crystalline lattice. The growth typically follows an initial stage of either homogeneous or heterogeneous (surface catalyzed) nucleation, unless a "seed" crystal, purposely added to start the growth, was already present. The action of crystal growth yields a crystalline solid whose atoms or molecules are close packed, with fixed positions in space relative to each other. The crystalline states of matter, state of matter is characterized by a distinct structural rigidity and very high resistance to Plastic deformation in solids, deformation (i.e. changes of shape and/or volume). Most crystalline solids have high values both of Young's modulus and of the shear modulus of elasticity (physics), elasticity. This contrasts with most liquids or fluids, which have a low shear modulus, and typically exh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supersaturation
In physical chemistry, supersaturation occurs with a solution (chemistry), solution when the concentration of a solute exceeds the concentration specified by the value of solubility at Solubility equilibrium, equilibrium. Most commonly the term is applied to a solution of a solid in a liquid, but it can also be applied to liquids and gases dissolved in a liquid. A supersaturated solution is in a metastable state; it may return to equilibrium by separation process, separation of the excess of solute from the solution, by dilution of the solution by adding solvent, or by increasing the solubility of the solute in the solvent. History Early studies of the phenomenon were conducted with sodium sulfate, also known as Glauber's Salt because, unusually, the solubility of this salt in water may decrease with increasing temperature. Early studies have been summarised by Tomlinson. It was shown that the crystallization of a supersaturated solution does not simply come from its agitation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) significantly below 0°C, it will tend to Freezing, freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In crystallography, polymorphism is the phenomenon where a compound or element can crystallize into more than one crystal structure. The preceding definition has evolved over many years and is still under discussion today. Discussion of the defining characteristics of polymorphism involves distinguishing among types of transitions and structural changes occurring in polymorphism versus those in other phenomena. Overview Phase transitions (phase changes) that help describe polymorphism include polymorphic transitions as well as melting and vaporization transitions. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." Additionally, Walter McCrone described the phases in polymorphic matter as "different in crystal structure but identical in the liquid or vapor states." McCrone also def ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. Per the established international definition, supercooling means ''‘cooling a substance below the normal freezing point without solidification’.'' IIR International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.php ASHRAE Terminology, https://www.ashrae.org/technical-resources/free-resources/ashrae-terminology While it can be achieved by different physical means, the postponed solidification is most often due to the absence of nucleation, seed crystals or nuclei around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to . Supercooled water can occur naturally, for example in the atmosphere, animals or plants. This phenomenon was first identified in 1724 by Daniel Gabriel Fahrenheit, while developing Fahren ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in a material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of principal axes/edges, of the unit cell and angles between them are lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of a crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freezing
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point. For most substances, the melting and freezing points are the same temperature; however, certain substances possess differing solid-liquid transition temperatures. For example, agar displays a Hysteresis#Liquid–solid-phase transitions, hysteresis in its melting point and freezing point. It melts at and solidifies from . Crystallization Most liquids freeze by crystallization, formation of crystal, crystalline solid from the uniform liquid. This is a first-order thermodynamic phase transition, which means that as long as solid and liquid coexist, the temperature of the whole system remains very nearly equal to the melting point due to the slow removal of heat when in contact with air, which is a poor heat conductor. Because of the latent heat of fusion, the freezing is greatly slowed and the temperature will not drop anymore once the freezing starts but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitation (chemistry)
In an aqueous solution, precipitation is the "sedimentation of a solid material (a precipitate) from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the supernate or supernatant. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (e.g. metallurgy and alloys) when solid impurities segregation (materials science), segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitation occurs more rapidly from a strongly supersaturated solution. The formation of a pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |