|
Claisen Ester Condensations
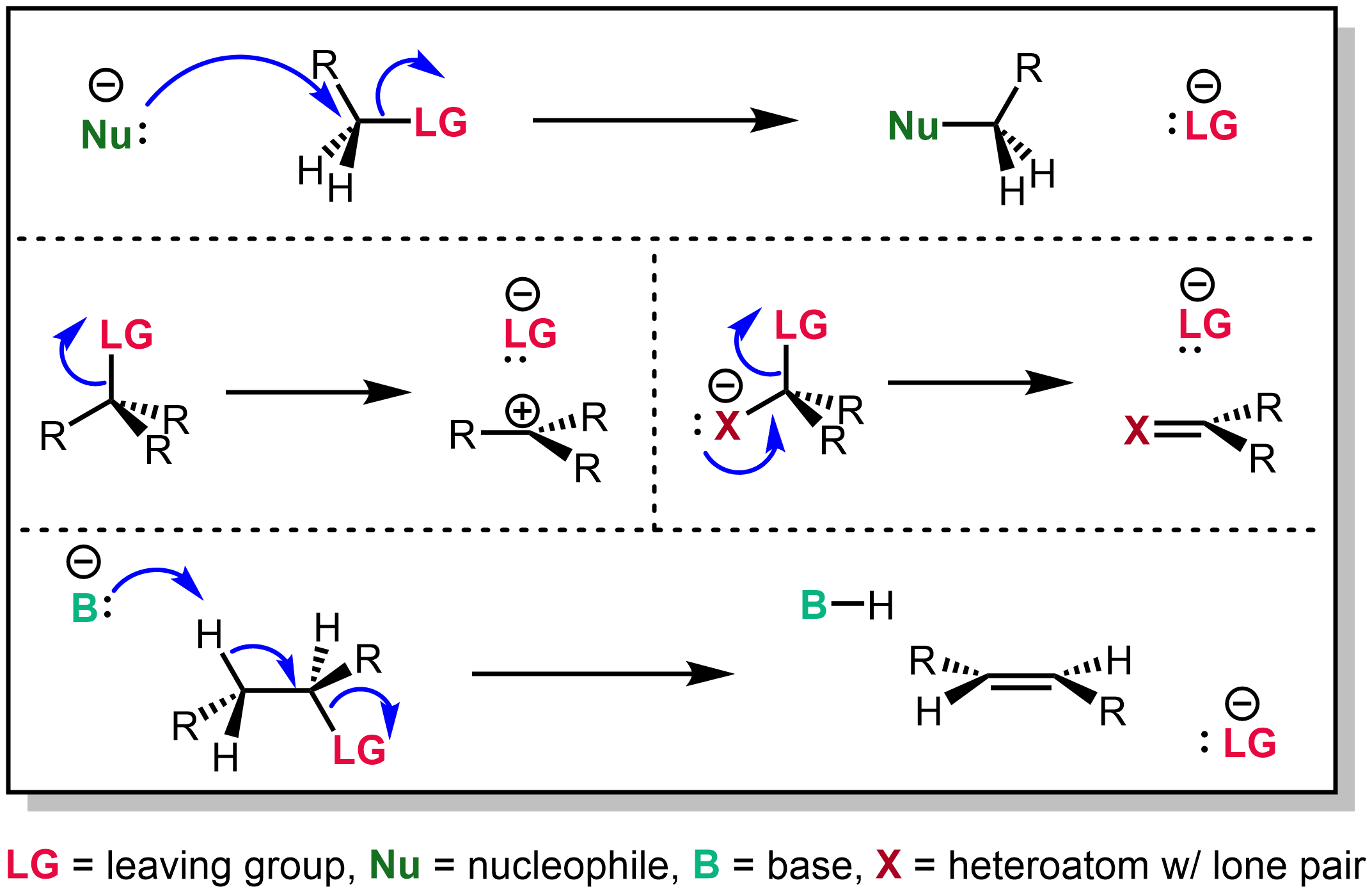
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base. The reaction produces a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. The reaction has often been displaced by diketene-based chemistry, which affords acetoacetic esters. Requirements At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rainer Ludwig Claisen
Rainer Ludwig Claisen (; 14 January 1851 – 5 January 1930) was a German chemist best known for his work with condensations of carbonyls and sigmatropic rearrangements. He was born in Cologne as the son of a jurist and studied chemistry at the university of Bonn (1869), where he became a member of K.St.V. Arminia. He served in the army as a nurse in 1870–1871 and continued his studies at Göttingen University. He returned to the University of Bonn in 1872 and started his academic career at the same university in 1874. He died in 1930 in Godesberg am Rhein (near Bonn). Career Scientific contributions * Described the condensation of aromatic aldehydes with aliphatic aldehydes or ketones in 1881. This variation of the now well-known aldol condensation reaction is called the Claisen–Schmidt condensation. * Discovered (1887) the condensation reaction of an ester with an activated methylene group, now known as the Claisen condensation. * Synthesis of cinnamates by rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule. Numeric locants The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of numeric prefixes to indicate the position of substituents, generally by identifying the parent hydrocarbon chain and assigning the carbon atoms based on their substituents in order of precedence. For example, there are at least two isomers of the linear form of pentanone, a ketone that contains a chain of exactly five carbon atoms. There is an oxygen atom bonded to one of the middle three carbons (if it were bonded to an end carbon, the molecule would be an aldehyde, not a ketone), but it is not clear where it is located. In this example, the carbon atoms are numbered from one to five, which starts at one end and proceeds sequentially along the chain. Now the position of the oxygen atom can be defined as on carbon atom number two, three o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through addition and substitution reactions. Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL. Organic chemistry Addition of halogens These occur between alkenes and electrophiles, often halogens as in halogen addition reactions. Common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
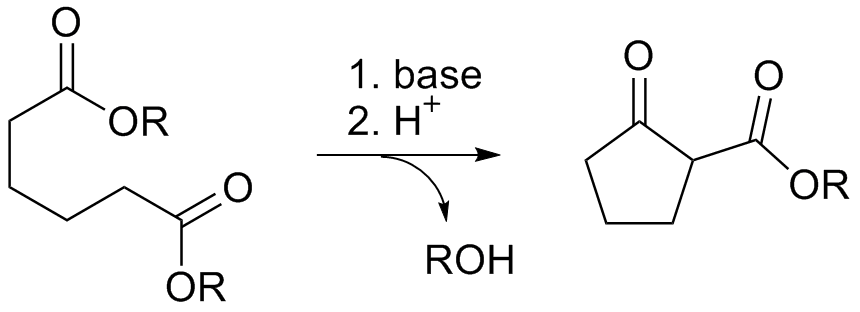
Dieckmann Condensation
The Dieckmann condensation is the Intramolecular reaction, intramolecular chemical reaction of ester, diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation. Dieckmann condensations are highly effective routes to 5-, 6-, and 7-member rings, but poor for larger rings. : Reaction mechanism Deprotonation of an ester at the α-position generates an enolate ion which then undergoes a Baldwin's rules, 5-exo-trig nucleophilic attack to give a cyclic enol. Protonation with a Brønsted–Lowry acid (H3O+ for example) re-forms the β-keto ester. : Due to the steric stability of five- and six-membered rings, these structures will preferentially be formed. 1,6 diesters will form five-membered cyclic β-keto esters, while 1,7 diesters will form six-membered β-keto esters. Further reading *Dieckmann, W. ''Chemische Berichte, Ber.'' 1894, ''27'', 102 & 965 *Dieckmann, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
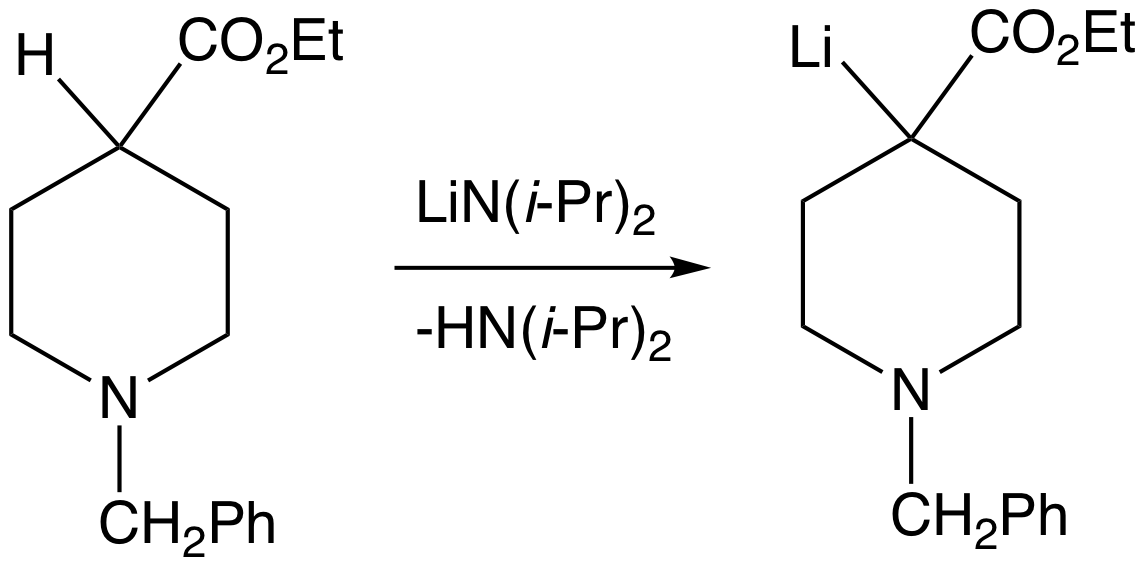
Lithium Diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group. Preparation and structure LDA is commonly formed by treating a cooled (0 to −78 °C) mixture of tetrahydrofuran and diisopropylamine with ''n''-butyllithium. When dissociated, the diisopropylamide anion can become protonated to form diisopropylamine. Diisopropylamine has a p''K''a value of 36. Therefore, its conjugate base is suitable for the deprotonation of compounds with greater acidity, importantly, such weakly acidic compounds (carbon acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-nucleophilic Base
As the name suggests, a non-nucleophilic base is a sterically hindered organic base that is a poor nucleophile. Normal bases are also nucleophiles, but often chemists seek the proton-removing ability of a base without any other functions. Typical non-nucleophilic bases are bulky, such that protons can attach to the basic center but alkylation and complexation is inhibited. Non-nucleophilic bases A variety of amines and nitrogen heterocycles are useful bases of moderate strength (pKa of conjugate acid around 10-13) * ''N'',''N''-Diisopropylethylamine (DIPEA, also called Hünig's Base), pKa = 10.75 *1,8-Diazabicycloundec-7-ene (DBU) - useful for E2 elimination reactions, pKa = 13.5 * 1,5-Diazabicyclo(4.3.0)non-5-ene (DBN) - comparable to DBU * 2,6-Di-tert-butylpyridine, a weak non-nucleophilic base pKa = 3.58 * Phosphazene bases, such as ''t''-Bu-P4''Activation in anionic polymerization: Why phosphazene bases are very exciting promoters'' S. Boileau, N. Illy Prog. Polym. Sci., 20 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Ethoxide
Sodium ethoxide, also referred to as sodium ethanolate, is the Ionic compound, ionic, organic compound with the formula , , or NaOEt (Et = ethyl group, ethyl). It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. It is commonly used as a strong Base (chemistry), base. Preparation Few procedures have been reported to prepare the anhydrous solid. Instead the material is typically prepared in a solution with ethanol. It is commercially available and as a solution in ethanol. It is easily prepared in the laboratory by treating sodium metal with absolute ethanol: : The reaction of sodium hydroxide with anhydrous ethanol suffers from incomplete conversion to the ethoxide, but can still produce dry NaOEt by precipitation using acetone, or by drying using additional NaOH. Structure The crystal structure of sodium ethoxide has been determined by X-ray crystallography. It consists of layers of alternating and centres with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis.excerpt Transition metal alkoxides are widely used for coatings and as catalysts. Enolates are unsaturated alkoxides derived by deprotonation of a bond adjacent to a ketone or aldehyde. The nucleophilic center for simple alkoxides is located on the oxygen, whereas the nucleophilic site on enolates is delocalized onto both carbon and oxygen sites. Ynolates are also unsaturated alkoxides derived from acetylenic alcohols. Phenoxides are close relatives of the alkoxides, in which the alkyl group is replaced by a phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs. Addition to carbon–heteroatom double bonds Nucleophilic addition reactions of nucleophiles with electrophilic double or triple bond (π bonds) create a new carbon center with two additional single, or σ, bonds.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Addition of a nucleophile to carbon–heteroatom double or triple bonds such as >C=O or -C≡N show great variety. These types of bonds are polar (have a large difference in electronegativit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |