|
Chloromethyl Group
In organic chemistry, the chloromethyl group is a functional group that has the chemical formula . The naming of this group is derived from the methyl group (which has the formula ), by replacing one hydrogen atom by a chlorine atom. Compounds with this group are a subclass of the organochlorines. The way of introducing a chloromethyl group into aromatic compounds is the chloromethylation by the Blanc reaction. See also * Trichloromethyl group The trichloromethyl group is a functional group that has the formula –CCl3. The naming of is group is derived from the methyl group (which has the formula –CH3), by replacing each hydrogen atom by a chlorine atom. Compounds with this group ar ... References {{organohalide-stub Organochlorides Functional groups ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloromethyl Group Expanded
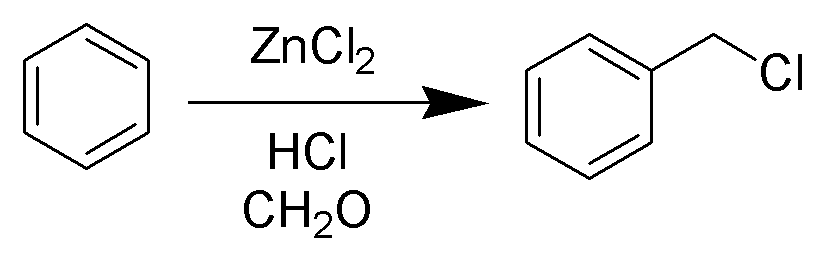
The Blanc chloromethylation (also called the Blanc reaction) is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923 Mechanism and scope The reaction is carried out under acidic conditions and with a ZnCl2 catalyst. These conditions protonate the formaldehyde carbonyl making the carbon much more electrophilic. The aldehyde is then attacked by the aromatic pi-electrons, followed by rearomatization of the aromatic ring. The benzyl alcohol thus formed is quickly converted to the chloride under the reaction conditions. Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride. These species may account for the fact that moderately and strongly deactivated substrates th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of ato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval alchemists, which commonly involved the heating of chloride salts like ammonium chloride ( sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However, the nature of free chlorine gas as a separate substance was only recognised ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organochlorine
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. Aliphatic organochlorides are often alkylating agents as chlorine can act as a leaving group, which can result in cellular damage. Natural occurrence Many organochlorine compounds have been isola ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties are understood. The current definition of aromatic compounds does not have any relation with their smell. Heteroarenes are closely related, since at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom. Hydrocarbons without an aromatic ring are called aliphatic. Benzene ring model Benzene, C6H6, is the least complex aromatic hydrocarbon, and it was the first one named as su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blanc Reaction
The Blanc chloromethylation (also called the Blanc reaction) is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923 Mechanism and scope The reaction is carried out under acidic conditions and with a ZnCl2 catalyst. These conditions protonate the formaldehyde carbonyl making the carbon much more electrophilic. The aldehyde is then attacked by the aromatic pi-electrons, followed by rearomatization of the aromatic ring. The benzyl alcohol thus formed is quickly converted to the chloride under the reaction conditions. Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride. These species may account for the fact that moderately and strongly deactivated substrates th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloromethyl Group
The trichloromethyl group is a functional group that has the formula –CCl3. The naming of is group is derived from the methyl group (which has the formula –CH3), by replacing each hydrogen atom by a chlorine atom. Compounds with this group are a subclass of the organochlorines. Some notable examples of compounds with this group are trichloromethane H–, 1,1,1-trichloroethane –, and chloral –. The trichloromethyl group has a significant electronegativity. For this reason, trichloromethyl-substituted acids, such as trichloromethanesulfonic acid, are often stronger than the original. For example, the acidity constant (pKa) of trichloroacetic acid – is 0.77, whereas that of acetic acid is 4.76. By the same principle, the trichloromethyl group generally lowers the basicity of organic compounds, e.g. trichloroethanol. See also * Trifluoromethyl group The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organochlorides
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. Aliphatic organochlorides are often alkylating agents as chlorine can act as a leaving group, which can result in cellular damage. Natural occurrence Many organochlorine compounds have been isolate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |