|
Chevreul's Salt
Chevreul's salt (copper(I,II) sulfite dihydrate, Cu2SO3•CuSO3•2H2O or Cu3(SO3)2•2H2O), is a copper salt which was prepared for the first time by a French chemist Michel Eugène Chevreul in 1812. Its unusual property is that it contains copper in both of its common oxidation states. It is insoluble in water and stable in air. What was known as Rogojski's salt is a mixture of Chevreul's salt and metallic copper. Preparation Chevreul's salt is prepared by treating aqueous copper sulfate with a solution of potassium metabisulfite. The solution changes colour from blue to green immediately. The identity of the green species is unknown. Heating this solution produces a reddish solid precipitate: 3 CuSO4 + 4 K2S2O5 + 3 H2O → Cu3(SO3)2•2H2O + 4 K2SO4 + 4 SO2 + H2SO4 When sodium ions are present in the solutions that form the salt, sodium can substitute for some of the copper (I), as the ions have the same charge and similar sizes. Reactions Chevreul's salt exhibits properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Near Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel discover ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Sulfate
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur. Uses The primary use of ammonium sulfate is as a fertilizer for alkaline soils. In the soil the ammonium ion is released and forms a small amount of acid, lowering the pH balance of the soil, while contributing essential nitrogen for plant growth. The main disadvantage to the use of ammonium sulfate is its low nitrogen content relative to ammonium nitrate, which elevates transportation costs.Karl-Heinz Zapp "Ammonium Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2012, Wiley-VCH, Weinheim. It is also used as an agricultural spray adjuvant for water-soluble insecticides, herbicides, and fungicides. There, it functions to bind iron and calcium cations that are present in both well water and pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrometallurgical Process
Hydrometallurgy is a technique within the field of extractive metallurgy, the obtaining of metals from their ores. Hydrometallurgy involve the use of aqueous solutions for the recovery of metals from ores, concentrates, and recycled or residual materials.Brent Hiskey "Metallurgy, Survey" in Kirk-Othmer Encyclopedia of Chemical Technology, 2000, Wiley-VCH, Weinheim. Processing techniques that complement hydrometallurgy are pyrometallurgy, vapour metallurgy, and molten salt electrometallurgy. Hydrometallurgy is typically divided into three general areas: *Leaching *Solution concentration and purification *Metal or metal compound recovery Leaching Leaching involves the use of aqueous solutions to extract metal from metal bearing materials which is brought into contact with a material containing a valuable metal. The first examples come from 11-12th centuries China where it was applied to extraction of copper and accounted for a significant share of total copper production. In the 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
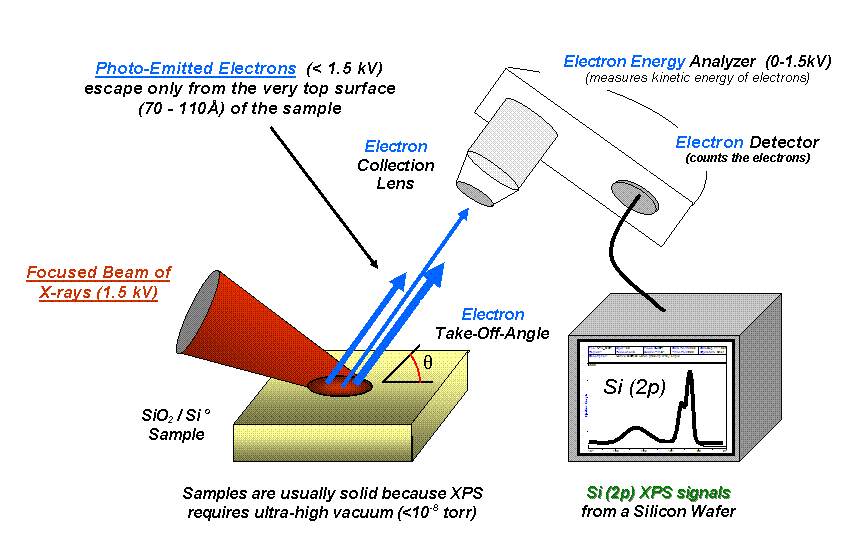
X-ray Photoelectron Spectrum
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material (elemental composition) or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation. XPS belongs to the family of photoemission spectroscopies in which electron population spectra are obtained by ir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Coordination
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix '' octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Susceptibility
In electromagnetism, the magnetic susceptibility (Latin: , "receptive"; denoted ) is a measure of how much a material will become magnetized in an applied magnetic field. It is the ratio of magnetization (magnetic moment per unit volume) to the applied magnetizing field intensity . This allows a simple classification, into two categories, of most materials' responses to an applied magnetic field: an alignment with the magnetic field, , called paramagnetism, or an alignment against the field, , called diamagnetism. Magnetic susceptibility indicates whether a material is attracted into or repelled out of a magnetic field. Paramagnetic materials align with the applied field and are attracted to regions of greater magnetic field. Diamagnetic materials are anti-aligned and are pushed away, toward regions of lower magnetic fields. On top of the applied field, the magnetization of the material adds its own magnetic field, causing the field lines to concentrate in paramagnetism, or be excl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Diffusivity
In heat transfer analysis, thermal diffusivity is the thermal conductivity divided by density and specific heat capacity at constant pressure. It measures the rate of transfer of heat of a material from the hot end to the cold end. It has the SI derived unit of m2/s. Thermal diffusivity is usually denoted by lowercase alpha (), but , , (kappa), , and are also used. The formula is: :\alpha = \frac where * is thermal conductivity (W/(m·K)) * is specific heat capacity (J/(kg·K)) * is density (kg/m3) Together, can be considered the volumetric heat capacity (J/(m3·K)). As seen in the heat equation, :\frac = \alpha \nabla^2 T, one way to view thermal diffusivity is as the ratio of the time derivative of temperature to its curvature, quantifying the rate at which temperature concavity is "smoothed out". In a sense, thermal diffusivity is a contrasting measure to thermal inertia. In a substance with high thermal diffusivity, heat moves rapidly through it because the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
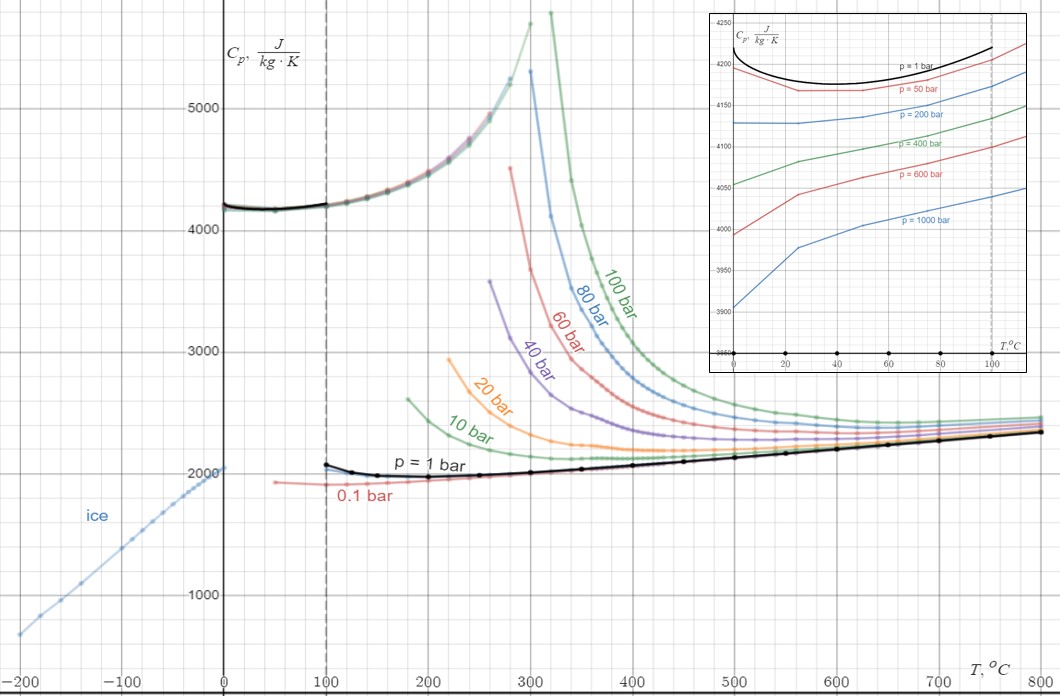
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its thermal mass. Definition Basic definition The heat capacity of an object, denoted by C, is the limit : C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials like Rockwool or Styrofoam. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla T is the temperature gradient. This is known as Fourier's Law for heat conduction. Although commonly expressed as a scalar, the most general form of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HSAB Theory
HSAB concept is a jargon for "hard and soft (Lewis) acids and bases". HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemical species. 'Hard' applies to species which are small, have high charge states (the charge criterion applies mainly to acids, to a lesser extent to bases), and are weakly polarizable. 'Soft' applies to species which are big, have low charge states and are strongly polarizable. The theory is used in contexts where a qualitative, rather than quantitative, description would help in understanding the predominant factors which drive chemical properties and reactions. This is especially so in transition metal chemistry, where numerous experiments have been done to determine the relative ordering of ligands and transition metal ions in terms of their hardness and softness. HSAB theory is also useful in predicting the products of metathesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomorphic Series
In chemistry isomorphism has meanings both at the level of crystallography and at a molecular level. In crystallography, compounds are isomorphous if their symmetry is the same and their unit cell parameters are similar Molecules are isomorphous if they have similar shapes. The coordination complexes tris(acetylacetonato)iron (Fe(acac)3) and tris(acetylacetonato)aluminium (Al(acac)3) are isomorphous. These compounds, both of Point groups in three dimensions#The seven infinite series of axial groups, ''D''3 symmetry have very similar shapes, as determined by bond lengths and bond angles. Isomorphous compounds give rise to isomorphous crystals and form solid solutions. Historically, crystal shape was defined by measuring the angles between crystal faces with a goniometer. Whereas crystals of Fe(acac)3 are deep red and crystals of Al(acac)3 are colorless, a solid solution of the two, i.e. Fe1−xAlx(acac)3 will be deep or pale pink depending on the Fe/Al ratio, x. Double salt, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |