|
Chelation
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity. The word ''chelation'' is derived from Greek χηλή, ''chēlē'', meaning "claw"; the ligands lie around the central atom like the claws of a crab. The term ''chelate'' () was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or ''chele'' (Greek) of the crab or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings." Chelation is useful in applications such as providing nutritional supplements, in chel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelation Therapy
Chelation therapy is a medical procedure that involves the administration of chelating agents to remove heavy metals from the body. Chelation therapy has a long history of use in clinical toxicology and remains in use for some very specific medical treatments, although it is administered under very careful medical supervision due to various inherent risks, including the mobilization of mercury and other metals through the brain and other parts of the body by the use of weak chelating agents that unbind with metals before elimination, exacerbating existing damage. To avoid mobilization, some practitioners of chelation use strong chelators, such as selenium, taken at low doses over a long period of time. Chelation therapy also has a history of fraudulent use in Alternative medicine, to treat claimed effects of heavy-metal exposure on problems as disparate as heart disease, cancer and autism. Chelation therapy must be administered with care as it has a number of possible side effec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylenediamine
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines. Synthesis Ethylenediamine is produced industrially by treating 1,2-dichloroethane with ammonia under pressure at 180 °C in an aqueous medium (EDC process): : In this reaction hydrogen chloride is generated, which forms a salt with the amine. The amine is liberated by addition of sodium hydroxide and can then be recovered by fractional distillation. Diethylenetriamine (DETA) and triethylenetetramine (TETA) are formed as by-products. Another industrial route to ethylenediamine involves the reaction of ethanolamine and ammonia:Hans-Jürgen Arpe, Industrielle Organische Chemie, 6. Auflage (2007), Seite 275, Wiley VCH : Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Denticity
In coordination chemistry, denticity () refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be unidentate or monodentate. Ligands with more than one bonded atom are called multidentate or polydentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms. Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used. Bridging ligands use the μ ('mu') notation. Classes Polydentate ligands are chelating agents and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or more binding si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stoichiometry
Stoichiometry () is the relationships between the masses of reactants and Product (chemistry), products before, during, and following chemical reactions. Stoichiometry is based on the law of conservation of mass; the total mass of reactants must equal the total mass of products, so the relationship between reactants and products must form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated. This is illustrated in the image here, where the unbalanced equation is: : : However, the current equation is imbalanced. The reactants have 4 hydrogen and 2 oxygen atoms, while the product has 2 hydrogen and 3 oxygen. To balance the hydrogen, a coefficient of 2 is added to the product H2O, and to fix the imbalance of oxygen, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylamine
Methylamine, also known as methanamine, is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. Methylamine is sold as a solution in methanol, ethanol, tetrahydrofuran, or water, or as the anhydrous gas in pressurized metal containers. Industrially, methylamine is transported in its anhydrous form in pressurized railcars and tank trailers. It has a strong odor similar to rotten fish. Methylamine is used as a building block for the synthesis of numerous other commercially available compounds. Industrial production Methylamine has been produced industrially since the 1920s (originally by Commercial Solvents Corporation for dehairing of animal skins). This was made possible by and his wife Eugenia who discovered amination of alcohols, including methanol, on alumina or kaolin catalyst after WWI, filed two patent applications in 1919 and published an a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equilibrium Thermodynamics
{{thermodynamics, cTopic=Branches Equilibrium Thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called thermodynamic equilibrium. The word equilibrium implies a state of balance. Equilibrium thermodynamics, in origins, derives from analysis of the Carnot cycle. Here, typically a system, as cylinder of gas, initially in its own state of internal thermodynamic equilibrium, is set ''out of balance'' via heat input from a combustion reaction. Then, through a series of steps, as the system settles into its final equilibrium state, work is extracted. In an equilibrium state the potentials, or driving forces, within the system, are in exact balance. A central aim in equilibrium thermodynamics is: given a system in a well-defined initial state of thermodynamic equilibrium, subject to accurately specified constraints, to calculate, when the constraints are changed by an externally imposed intervention, what the state ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equilibrium Constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further change. For a given set of reaction conditions, the equilibrium constant is independent of the initial analytical concentrations of the reactant and product species in the mixture. Thus, given the initial composition of a system, known equilibrium constant values can be used to determine the Chemical equilibrium#Composition of a mixture, composition of the system at equilibrium. However, reaction parameters like temperature, solvent, and ionic strength may all influence the value of the equilibrium constant. A knowledge of equilibrium constants is essential for the understanding of many chemical systems, as well as the biochemical processes such as oxygen transport by hemoglobin in blood and acid–base homeostasis in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
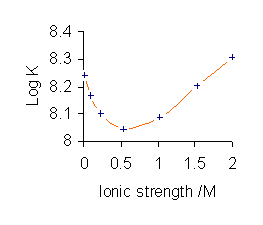
Stability Constants Of Complexes
In coordination chemistry, a stability constant (also called formation constant or binding constant) is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the Complex (chemistry), complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine. History Jannik Bjerrum (son of Niels Bjerrum) developed the first general method for the determination of stability constants of Metal ammine complex, metal-ammine complexes in 1941. The reasons why this occurred at such a late date, nearly 50 years after Alfred Werner had proposed the correct structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ (potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− (chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indicates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Concentration
Molar concentration (also called molarity, amount concentration or substance concentration) is the number of moles of solute per liter of solution. Specifically, It is a measure of the concentration of a chemical species, in particular, of a solute in a solution, in terms of amount of substance per unit volume of solution. In chemistry, the most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol/L or mol/ dm3 (1000 mol/ m3) in SI units. A solution with a concentration of 1 mol/L is said to be 1 molar, commonly designated as 1 M or 1 M. Molarity is often depicted with square brackets around the substance of interest; for example, the molarity of the hydrogen ion is depicted as + Definition Molar concentration or molarity is most commonly expressed in units of moles of solute per litre of solution. For use in broader applications, it is defined as amount of substance of solute per unit volume of solution, or pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable, unalloyed metallic form. This means that copper is a native metal. This led to very early human use in several regions, from . Thousands of years later, it was the first metal to be smelted from sulfide ores, ; the first metal to be cast into a shape in a mold, ; and the first metal to be purposely alloyed with another metal, tin, to create bronze, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |