|
Catalytic Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons. Process Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same catalysts and conditions that are used for hydrogenation reactions can also lead to isomerization of the alkenes fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petrochemical Industry
file:Jampilen Petrochemical Co. 02.jpg, 300px, Jampilen Petrochemical co., Asaluyeh, Iran The petrochemical industry is concerned with the production and trade of petrochemicals. A major part is constituted by the plastics industry, plastics (polymer) industry. It directly interfaces with the petroleum industry, especially the downstream (petroleum industry), downstream sector. Companies The top global petrochemical companies based on different KPIs: Countries and sites *Marun petrochemical complex Technology Conferences *Asia Petrochemical Industry Conference *International Petrochemical Conference by the American Fuel and Petrochemical Manufacturers, AFPM Associations *American Fuel and Petrochemical Manufacturers (AFPM) *European Petrochemical Association (EPCA) *Gulf Petrochemicals and Chemicals Association (GPCA) *Petrochemicals Europe (industry sector of Cefic) Awards *Medal "For the Tapping of the Subsoil and Expansion of the Petrochemical Complex of Western Siberia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, single bonds or double bond, double bonds, with a bond order of three. The most common triple bond is in a nitrogen N2 molecule; the second most common is that between two carbon atoms, which can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as diphosphorus and carbon monoxide, are also triple bonded. In skeletal formula, skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms. Bonding Triple bonding can be explained in terms of orbital hybridization. In the case of acetylene, each carbon atom has two sp orbital, sp-orbitals and two p-orbitals. The two sp-orbitals are linear, with 180° bond angles, and occupy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable, organic compound with a pungent alcoholic odor. Isopropyl alcohol, an organic polar molecule, is miscible in water, ethanol, and chloroform, demonstrating its ability to dissolve a wide range of substances including ethyl cellulose, polyvinyl butyral, oils, alkaloids, and natural resins. Notably, it is not miscible with salt solutions and can be separated by adding sodium chloride in a process known as salting out. It forms an azeotrope with water, resulting in a boiling point of 80.37 °C and is characterized by its slightly bitter taste. Isopropyl alcohol becomes viscous at lower temperatures, freezing at −89.5 °C, and has significant ultraviolet-visible absorbance at 205 nm. Chemically, it can be oxidized to acetone or undergo various reactions to form compounds like isopropoxides or aluminium isopropoxide. As an isopropyl group linked ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formic Acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts, and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol. Natural occurrence Formic acid, which has a pungent, penetrating odor, is found naturally in insects, weeds, fruits and vegetables, and forest emissions. It appears in most ants and in stingless bees of the genus '' Oxytrigona''. Wood ants from the genus ''Formica'' can spray formic acid on their prey or to defend the nest. The puss moth caterpillar (''Cerura vinula'') will spray it as well when threatened by predators. It is also found in the trichomes of stinging nettle (''Urtica dioica''). Apart from that, this acid is incorporated in many fruits such as pineapple (0.21 mg per 100 g), apple (2 mg per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
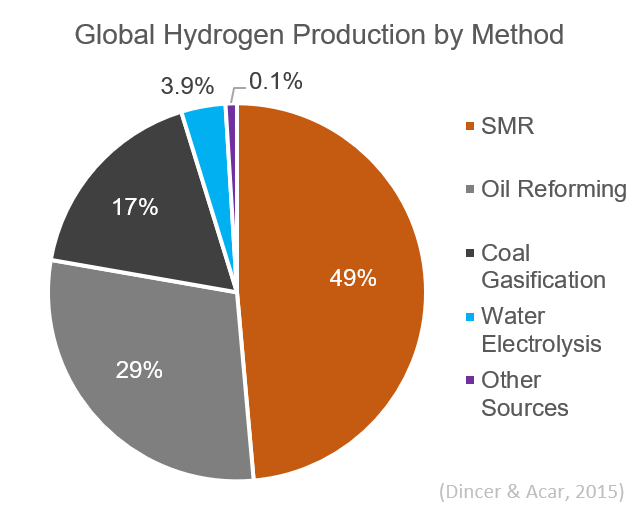
Steam Reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly, natural gas is the feedstock. The main purpose of this technology is often hydrogen production, although syngas has multiple other uses such as production of Ammonia production, ammonia or methanol. The reaction is represented by this equilibrium: :CH4 + H2O CO + 3 H2 The reaction is strongly endothermic (Δ''H''SR = 206 kJ/mol). Hydrogen produced by steam reforming is termed grey hydrogen, 'grey' hydrogen when the waste carbon dioxide is released to the atmosphere and blue hydrogen, 'blue' hydrogen when the carbon dioxide is (mostly) captured and stored geologically—see carbon capture and storage. Zero carbon green hydrogen, 'green' hydrogen is produced by Thermochemical cycle, thermochemical water splitting, using solar thermal, low- or zero-carbon electricity or waste heat, or electrolysis, using low- or zero-carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" or "salt maker". When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room temperature the same becomes true of groups 1 and 15, assuming white phosphorus is taken as the standard state.This could also be the case for group 12, al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc.; 1932; 54(12); 4678–4690. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without cleaving bonds. Usually hydrogenolysis is conducted catalytically using hydrogen gas. History The term "hydrogenolysis" was coined by Carleton Ellis in reference to hydrogenolysis of carbon–carbon bonds. Earlier, Paul Sabatier had already observed the hydrogenolysis of benzyl alcohol to toluene, and as early as 1906, Padoa and Ponti had observed the hydrogenolysis of furfuryl alcohol. Homer Burton Adkins and Ralph Connor were the first to call the carbon–oxygen bond cleavage "hydrogenolysis". In the petrochemical industry In petroleum refineries ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans Fat
Trans fat is a type of unsaturated fat that occurs in foods. Small amounts of trans fats occur naturally, but large amounts are found in some processed foods made with partially hydrogenated oils. Because consumption of trans fats is associated with increased risk for cardiovascular diseases, artificial trans fats are highly regulated or banned in many countries. However, they are still widely consumed in developing nations where they are associated with increased risk of diabetes, cardiovascular diseases, and death. In 2015, the US Food and Drug Administration (FDA) stated that artificial trans fats from partially hydrogenated oils were not generally recognized as safe (GRAS), and the use of such oils and trans fats should be limited or eliminated from manufactured foods. Numerous governing bodies, including the European Union, Canada, and Australia/New Zealand, followed with restrictions or bans on the use of partially hydrogenated oils and trans fats in food manufacturing. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis–trans Isomerism
''Cis''–''trans'' isomerism, also known as geometric isomerism, describes certain arrangements of atoms within molecules. The prefixes "''cis''" and "''trans''" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, ''cis'' indicates that the functional groups (substituents) are on the same side of some plane, while ''trans'' conveys that they are on opposing (transverse) sides. ''Cis''–''trans'' isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. ''Cis'' and ''trans'' isomers occur both in organic molecules and in inorganic coordination complexes. ''Cis'' and ''trans'' descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "''syn''" and "''anti''" are used. According to IUPAC, "geome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |