|
Carrel–Dakin Method
Dakin's solution is a dilute solution of sodium hypochlorite (0.4% to 0.5%) and other stabilizing ingredients, traditionally used as an antiseptic, e.g. to cleanse wounds in order to prevent infection.Jeffrey M. Levine (2013): "Dakin’s Solution: Past, Present, and Future". ''Advances in Skin & Wound Care: The Journal for Prevention and Healing'', volume 26, issue 9, pp. 410–414. The preparation was for a time called also Carrel–Dakin solution or Carrel–Dakin fluid. Use Carrel and Dakin used a variety of apparatuses to infuse the solution continuously over the wounds. In modern typical usage, the solution is applied to the wound once daily for lightly to moderately exudative wounds, and twice daily for heavily exudative wounds or highly contaminated wounds.Century Pharmaceuticals, Inc.:Dakin's solution FAQ. Accessed 2018-06-14. The healthy skin surrounding the wound should preferably be protected with a moisture barrier ointment (e.g., petroleum jelly) or skin sealant as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hypochlorite
Sodium hypochlorite is an alkaline inorganic chemical compound with the formula (also written as NaClO). It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations () and hypochlorite anions (, also written as and ). The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate , a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated. Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as chlorine bleach, which is a household chemical widely used (since the 18th century) as a disinfectant and bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is still the most important chlorine-based bleach. Its corrosive properties, common availability, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
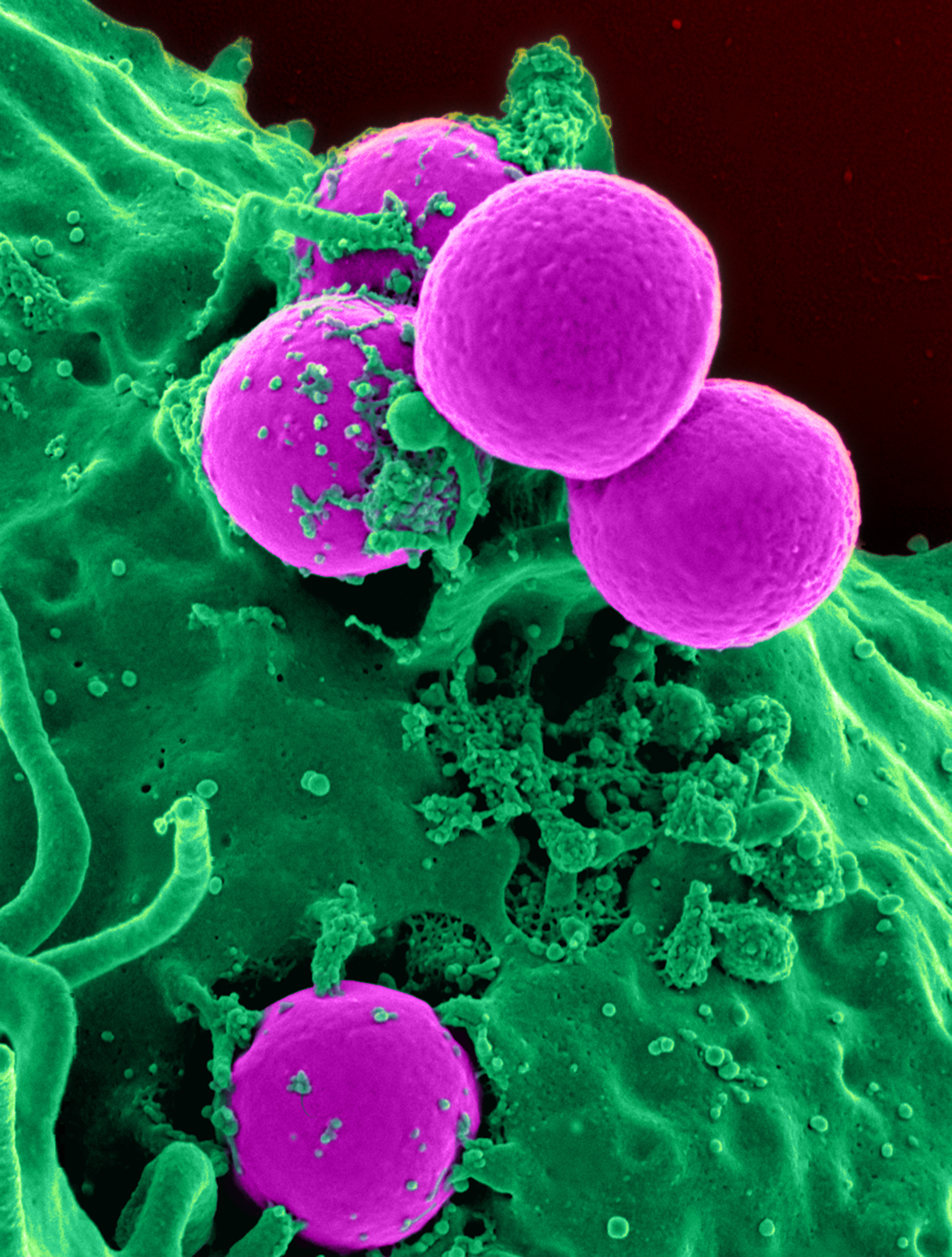
Asepsis
Asepsis is the state of being free from disease-causing micro-organisms (such as pathogenic bacteria, viruses, pathogenic fungi, and parasites). There are two categories of asepsis: medical and surgical. The modern day notion of asepsis is derived from the older antiseptic techniques, a shift initiated by different individuals in the 19th century who introduced practices such as the sterilizing of surgical tools and the wearing of surgical gloves during operations. The goal of asepsis is to eliminate infection, not to achieve sterility. Ideally, an operating field is sterile, meaning it is free of all biological contaminants (e.g. fungi, bacteria, viruses), not just those that can cause disease, putrefaction, or fermentation. Even in an aseptic state, a condition of sterile inflammation may develop. The term often refers to those practices used to promote or induce asepsis in an operative field of surgery or medicine to prevent infection. History The modern concept of asepsis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalinity
Alkalinity (from ) is the capacity of water to resist Freshwater acidification, acidification. It should not be confused with base (chemistry), basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate acid, conjugate bases. It is measured by Titration, titrating the Solution (chemistry), solution with an acid such as HCl until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (milliequivalents per liter), μeq/kg (microequivalents per kilogram), or mg/L CaCO3 (milligrams per liter of calcium carbonate). Each of these measurements corresponds to an amount of acid added as a titrant. In Fresh water, freshwater, particularly those on non-limestone terrains, alkalinities are low and involve a lot of ions. In the ocean, on the other hand, alkalinity is completely dominated by carbonate and bicarbonate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffering Agent
A buffer solution is a solution where the pH does not change significantly on dilution or if an acid or base is added at constant temperature. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion concentration increas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boric Acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salt (chemistry), salts, and can react with Alcohol (chemistry), alcohols to form borate esters. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds. The term "boric acid" is also used generically for any oxyacid of boron, such as metaboric acid and tetraboric acid . History Orthoboric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name ("sedative salt of Homberg"). However, boric acid and borates have been used since the time of the ancient Greece, anc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Chemistry Calcium carbonate shares the typical properties of other carbonates. Notably, it: *reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water: : *releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 840 °C in the case of ), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Carbonate
Sodium carbonate (also known as washing soda, soda ash, sal soda, and soda crystals) is the inorganic compound with the formula and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate ( natron), Na2CO3·10H2O, which readily effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate ( thermonatr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hypochlorite
Calcium hypochlorite is an inorganic compound with chemical formula , also written as . It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical. It is the main active ingredient of commercial products called bleaching powder, used for water treatment and as a bleaching agent. History Charles Tennant and Charles Macintosh developed an industrial process in the late 18th century for the manufacture of chloride of lime, patenting it in 1799. Tennant's process is essentially still used today, and became of military importance during World War I, because calcium hypochlorite was the active ingredient in trench disinfectant. Uses Sanitation Calcium hypochlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Bleach
Liquid bleach, often called just bleach, is a common chemical household product that consists of a dilute solution of sodium hypochlorite () and other secondary ingredients. It is a chlorine releasing bleaching agent widely used to whiten clothes and remove stains, as a disinfectant to kill germs, and for several other uses. While the term has had this meaning for a long time, it may now be applied more generically to any liquid bleaching agent for laundry, irrespective of composition, such as peroxide-based bleaches. History Potassium hypochlorite () was synthesized by French scientist Berthollet in 1789, by reacting chlorine gas () with a solution of potassium hydroxide (potash, ). He also discovered its cloth bleaching properties, and set out to commercialize it under the name of ''Eau de Javel'' ("water of Javel") after the borough of Paris where it was manufactured. It was the first product intended specifically for that application, and it shortened the process of bleac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. rubens''. Most penicillins in clinical use are synthesised by ''Penicillium chrysogenum, P. chrysogenum'' using industrial fermentation, deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: benzylpenicillin, penicillin G (intramuscular injection, intramuscular or Intravenous therapy, intravenous use) and phenoxymethylpenicillin, penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococcus, staphylococci and streptococcus, streptococci. They are still widely used today for various bacterial infections, though many types of bacteria have developed antibiotic res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eugenics
Eugenics is a set of largely discredited beliefs and practices that aim to improve the genetic quality of a human population. Historically, eugenicists have attempted to alter the frequency of various human phenotypes by inhibiting the fertility of those considered inferior, or promoting that of those considered superior. The contemporary history of eugenics began in the late 19th century, when a popular eugenics movement emerged in the United Kingdom, and then spread to many countries, including the United States, Canada, Australia, and most European countries (e.g. Sweden and Germany). In this period, people from across the political spectrum espoused eugenics. Many countries adopted eugenic policies intended to improve the quality of their populations. Historically, the idea of ''eugenics'' has been used to argue for a broad array of practices ranging from prenatal care for mothers deemed genetically desirable to the forced sterilization and murder of those deemed unf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |