|
CANX
Calnexin (CNX) is a 67kDa integral protein (that appears variously as a 90kDa, 80kDa, or 75kDa band on western blotting depending on the source of the antibody) of the endoplasmic reticulum (ER). It consists of a large (50 kDa) N-terminal calcium- binding lumenal domain, a single transmembrane helix and a short (90 residues), acidic cytoplasmic tail. In humans, calnexin is encoded by the gene ''CANX''. Function Calnexin is a chaperone, characterized by assisting protein folding and quality control, ensuring that only properly folded and assembled proteins proceed further along the secretory pathway. It specifically acts to retain unfolded or unassembled N-linked glycoproteins in the ER. Calnexin binds only those ''N''-glycoproteins that have GlcNAc2Man9Glc1 oligosaccharides. These monoglucosylated oligosaccharides result from the trimming of two glucose residues by the sequential action of two glucosidases, I and II. Glucosidase II can also remove the third and last gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
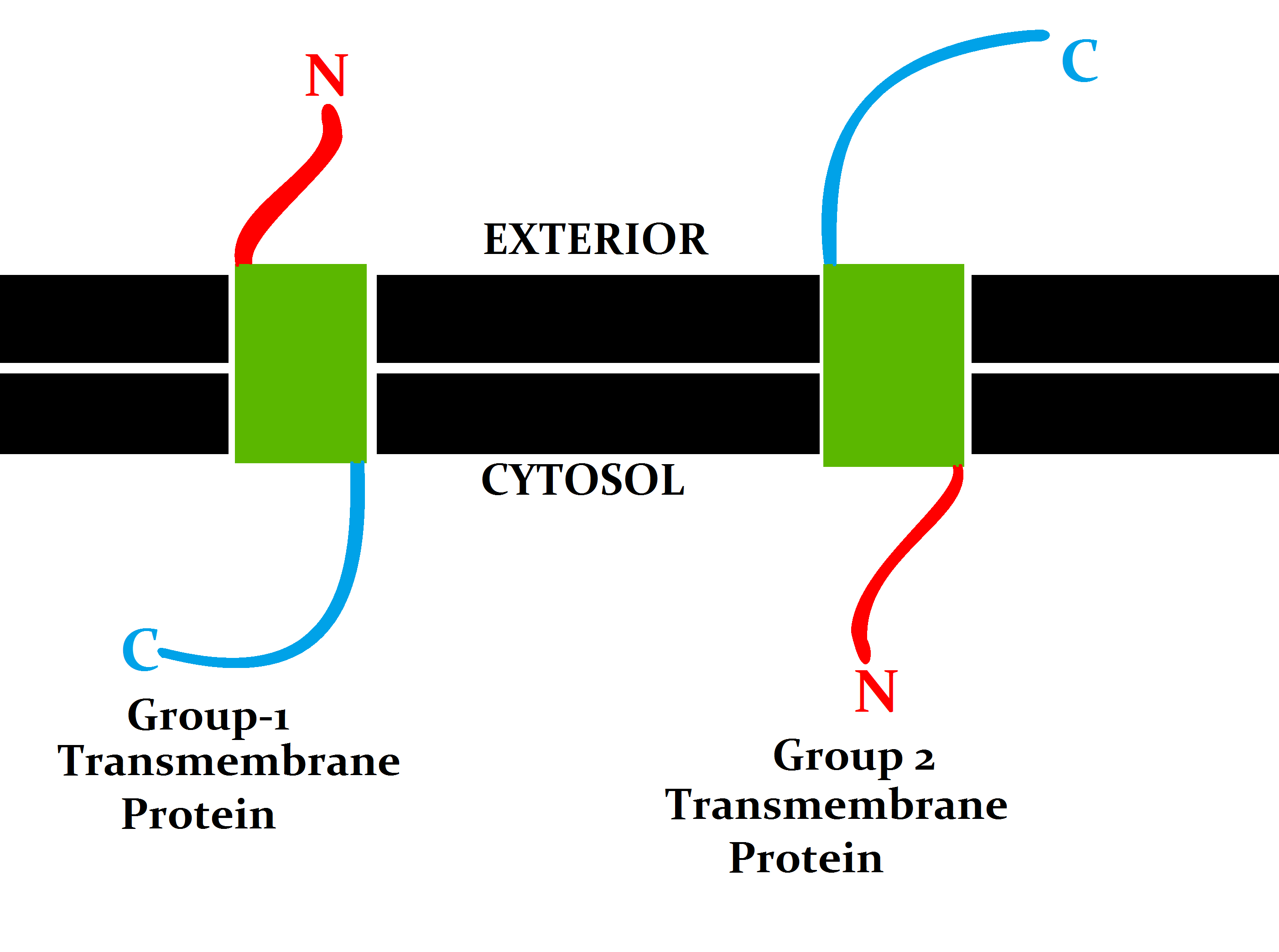
Integral Protein
An integral, or intrinsic, membrane protein (IMP) is a type of membrane protein that is permanently attached to the biological membrane. All transmembrane proteins can be classified as IMPs, but not all IMPs are transmembrane proteins. IMPs comprise a significant fraction of the proteins encoded in an organism's genome. Proteins that cross the membrane are surrounded by annular lipids, which are defined as lipids that are in direct contact with a membrane protein. Such proteins can only be separated from the membranes by using detergents, nonpolar solvents, or sometimes denaturing agents. Proteins that adhere only temporarily to cellular membranes are known as peripheral membrane proteins. These proteins can either associate with integral membrane proteins, or independently insert in the lipid bilayer in several ways. Structure Three-dimensional structures of ~160 different integral membrane proteins have been determined at atomic resolution by X-ray crystallography or nuclear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucosidase
Glucosidases are the glycoside hydrolase enzymes categorized under the EC number 3.2.1. Function Alpha-glucosidases are enzymes involved in breaking down complex carbohydrates such as starch and glycogen into their monomers. They catalyze the cleavage of individual glucosyl residues from various glycoconjugates including alpha- or beta-linked polymers of glucose. This enzyme convert complex sugars into simpler ones. Members Different sources include different members in this class. Members marked with a "#" are considered by MeSH to be glucosidases. Clinical significance Alpha-glucosidases are targeted by alpha-glucosidase inhibitors such as acarbose and miglitol to control diabetes mellitus type 2 Type 2 diabetes (T2D), formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent .... See also * DNA glyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ca++
Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization. Many enzymes require calcium ions as a cofactor, including several of the coagulation factors. Extracellular calcium is also important for maintaining the potential difference across excitable cell membranes, as well as proper bone formation. Plasma calcium levels in mammals are tightly regulated, electronic-book electronic- with bone acting as the major mineral storage site. Calcium ions, Ca2+, are released from bone into the bloodstream under controlled conditions. Calcium is transported through the bloodstream as dissolved ions or bound to proteins such as serum albumin. Parathyroid hormone secreted by the parathyroid gland regulates the resorption of Ca2+ from bone, reabsorption in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" for intracellular energy transfer. When consumed in a Metabolism, metabolic process, ATP converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. It is also a Precursor (chemistry), precursor to DNA and RNA, and is used as a coenzyme. An average adult human processes around 50 kilograms (about 100 mole (unit), moles) daily. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the Polyphosphate, triphosphate. Structure ATP consists of three parts: a sugar, an amine base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteasome
Proteasomes are essential protein complexes responsible for the degradation of proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases. Proteasomes are found inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, proteasomes are located both in the nucleus and in the cytoplasm. The proteasomal degradation pathway is essential for many cellular processes, including the cell cycle, the regulation of gene expression, and responses to oxidative stress. The importance of proteolytic degradation inside cells and the role of ubiquitin in proteolytic pathways was acknowledged in the award of the 2004 Nobel Prize in Chemistry to Aaron Ciechanover, Avram Hershko and Irwin Rose. The core 20S proteasome (blue in the adjacent figure) is a cylindrical, compartmental protein complex of four stacked rings forming a central pore. Each ring is composed of seven individual proteins. The inner two rings a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A. The addition of ubiquitin to a substrate protein is called ubiquitylation (or ubiquitination or ubiquitinylation). Ubiquitylation affects proteins in many ways: it can mark them for degradation via the 26S proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitylation involves three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade is to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myelination
Myelination, or myelinogenesis, is the formation and development of myelin sheaths in the nervous system, typically initiated in late prenatal neurodevelopment and continuing throughout postnatal development. The term ''myelinogenesis'' is also sometimes used to differentiate the very early stages of embryonic myelination. Myelin is formed by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. Myelination continues throughout the lifespan to support learning and memory via neural circuit plasticity as well as remyelination following injury. Successful myelination of axons increases action potential speed by enabling saltatory conduction, which is essential for timely signal conduction between spatially separate brain regions, as well as provides metabolic support to neurons. Stages Myelin is formed by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. Therefore, the first stage of m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schwann Cell
Schwann cells or neurolemmocytes (named after German physiologist Theodor Schwann) are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include Satellite glial cell, satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are Myelin, myelinating and Nonmyelinating Schwann cell, nonmyelinating. Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the Upstream and downstream (DNA), downstream region of the human dystrophin gene that gives shortened Transcription (biology), transcript that are again synthesized in a tissue-specific manner. During the development of the PNS, the regulatory mechanisms of myelination are controlled by feedforward interaction of specific genes, influencing transcriptional cascades and sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charcot–Marie–Tooth Disease
Charcot-Marie-Tooth disease (CMT) is an inherited neurological disorder that affects the peripheral nerves responsible for transmitting signals between the brain, spinal cord, and the rest of the body. This is the most common inherited neuropathy that causes sensory and motor symptoms of numbness, tingling, weakness and muscle atrophy, pain, and progressive foot deformities over time. In some cases, CMT also affects nerves controlling automatic bodily functions like sweating and balance. Symptoms typically start in the feet and legs before spreading to the hands and arms. While some individuals experience minimal symptoms, others may face significant physical limitations. There is no cure for CMT; however, treatments such as physical therapy, orthopedic devices, surgery, and medications can help manage symptoms and improve quality of life. CMT is caused by mutations in over 100 different genes, which disrupt the function of nerve cells' axons (responsible for transmitting signals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PMP22
Peripheral myelin protein 22 (PMP22), also called Growth arrest-specific protein 3 (GAS-3), is a protein which in humans is encoded by the ''PMP22'' gene. Mutations in ''PMP22'' cause changes in the expression of peripheral myelin protein 22 which can result in several neuropathies. PMP22 is a 22 kDa transmembrane glycoprotein made up of 160 amino acids, and is mainly expressed in the Schwann cells of the peripheral nervous system. Schwann cells show high expression of PMP22, where it can constitute 2-5% of total protein content in compact myelin. Compact myelin is the bulk of the peripheral neuron's myelin sheath, a protective fatty layer that provides electrical insulation for the neuronal axon. The level of PMP22 expression is relatively low in the central nervous system of adults. Like other membrane proteins, newly translated PMP22 protein is temporarily sequestered to the endoplasmic reticulum (ER) and Golgi apparatus for post-translational modifications. PMP22 protein is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transporter Associated With Antigen Processing
Transporter associated with antigen processing (TAP) protein complex belongs to the ATP-binding-cassette transporter family. It delivers cytosolic peptides into the endoplasmic reticulum (ER), where they bind to nascent MHC class I molecules. The TAP structure is formed of two proteins: TAP-1 and TAP-2, which have one hydrophobic region and one ATP-binding region each. They assemble into a heterodimer, which results in a four-domain transporter. Function The TAP transporter is found in the ER lumen associated with the peptide-loading complex (PLC). This complex of β2 microglobulin, calreticulin, ERp57, TAP, tapasin, and MHC class I acts to keep hold of MHC molecules until they have been fully loaded with peptides. Peptide transport TAP-mediated peptide transport is a multistep process. The peptide-binding pocket is formed by TAP-1 and TAP-2. Association with TAP is an ATP-independent event, ‘in a fast bimolecular association step, peptide binds to TAP, followed by a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MHC Class I
MHC class I molecules are one of two primary classes of major histocompatibility complex (MHC) molecules (the other being MHC class II) and are found on the cell surface of all nucleated cells in the bodies of vertebrates. They also occur on platelets, but not on red blood cells. Their function is to display peptide fragments of proteins from within the cell to cytotoxic T cells; this will trigger an immediate response from the immune system against a particular non-self antigen displayed with the help of an MHC class I protein. Because MHC class I molecules present peptides derived from cytosolic proteins, the pathway of MHC class I presentation is often called ''cytosolic'' or ''endogenous pathway''. In humans, the HLAs corresponding to MHC class I are HLA-A, HLA-B, and HLA-C. Function Class I MHC molecules bind peptides generated mainly from the degradation of cytosolic proteins by the proteasome. The MHC I: peptide complex is then inserted via the endoplasmic re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |