|
Calcium Hexaboride
Calcium hexaboride (sometimes calcium boride) is a compound of calcium and boron with the chemical formula CaB6. It is an important material due to its high electrical conductivity , hardness, chemical stability, and melting point. It is a black, lustrous, chemically inert powder with a low density. It has the cubic structure typical for metal hexaborides, with octahedral units of 6 boron atoms combined with calcium atoms. CaB6 and lanthanum-doped CaB6 both show weak ferromagnetic properties, which is a remarkable fact because calcium and boron are neither magnetic, nor have inner 3d or 4f electronic shells, which are usually required for ferromagnetism. Properties CaB6 has been investigated in the past due to a variety of peculiar physical properties, such as superconductivity, valence fluctuation and Kondo effects.. However, the most remarkable property of CaB6 is its ferromagnetism. It occurs at unexpectedly high temperature (600 K) and with low magnetic moment (below 0.07 \mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond. History Boron carbide was discovered in the 19th century as a by-product of reactions involving metal borides, but its chemical formula was unknown. It was not until the 1930s that the chemical composition was estimated as B4C. Controversy remained as to whether or not the material had this exact 4:1 stoichiometry, as, in practice the material is always slightly carbon-deficient with regard to this formula, and X-ray crystallography shows that its structure is highly complex, with a mixture of C-B-C chains and B12 icosahedra. These features argued against a very simple exact B4C empirical formula. Because of the B12 structural unit, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boride
A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some borides exhibit very useful physical properties. The term boride is also loosely applied to compounds such as B12As2 (N.B. Arsenic has an electronegativity higher than boron) that is often referred to as icosahedral boride. Ranges of compounds The borides can be classified loosely as boron rich or metal rich, for example the compound YB66 at one extreme through to Nd2Fe14B at the other. The generally accepted definition is that if the ratio of boron atoms to metal atoms is 4:1 or more, the compound is boron rich; if it is less, then it is metal rich. Boron rich borides (B:M 4:1 or more) The main group metals, lanthanides and actinides form a wide variety of boron-rich borides, with metal:boron ratios up to YB66. The properties of this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoelectric
The thermoelectric effect is the direct conversion of temperature differences to electric voltage and vice versa via a thermocouple. A thermoelectric device creates a voltage when there is a different temperature on each side. Conversely, when a voltage is applied to it, heat is transferred from one side to the other, creating a temperature difference. This effect can be used to generate electricity, measure temperature or change the temperature of objects. Because the direction of heating and cooling is affected by the applied voltage, thermoelectric devices can be used as temperature controllers. The term "thermoelectric effect" encompasses three separately identified effects: the Seebeck effect (temperature differences cause electromotive forces), the Peltier effect (thermocouples create temperature differences), and the Thomson effect (the Seebeck coefficient varies with temperature). The Seebeck and Peltier effects are different manifestations of the same physical proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
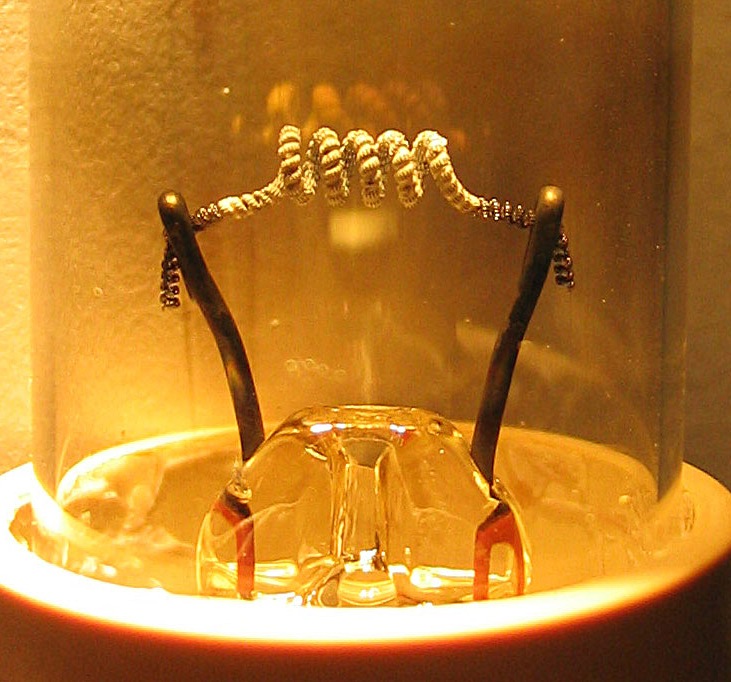
Hot Cathode
In vacuum tubes and gas-filled tubes, a hot cathode or thermionic cathode is a cathode electrode which is heated to make it emit electrons due to thermionic emission. This is in contrast to a cold cathode, which does not have a heating element. The heating element is usually an electrical filament heated by a separate electric current passing through it. Hot cathodes typically achieve much higher power density than cold cathodes, emitting significantly more electrons from the same surface area. Cold cathodes rely on field electron emission or secondary electron emission from positive ion bombardment, and do not require heating. There are two types of hot cathode. In a ''directly heated cathode'', the filament is the cathode and emits the electrons. In an ''indirectly heated cathode'', the filament or ''heater'' heats a separate metal cathode electrode which emits the electrons. From the 1920s to the 1960s, a wide variety of electronic devices used hot-cathode vacuum tubes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work Function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" means that the final electron position is far from the surface on the atomic scale, but still too close to the solid to be influenced by ambient electric fields in the vacuum. The work function is not a characteristic of a bulk material, but rather a property of the surface of the material (depending on crystal face and contamination). Definition The work function for a given surface is defined by the difference :W = -e\phi - E_, where is the charge of an electron, is the electrostatic potential in the vacuum nearby the surface, and is the Fermi level (electrochemical potential of electrons) inside the material. The term is the energy of an electron at rest in the vacuum nearby the surface. In practice, one directly controls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abrasive
An abrasive is a material, often a mineral, that is used to shape or finish a workpiece through rubbing which leads to part of the workpiece being worn away by friction. While finishing a material often means polishing it to gain a smooth, reflective surface, the process can also involve roughening as in satin, matte or beaded finishes. In short, the ceramics which are used to cut, grind and polish other softer materials are known as abrasives. Abrasives are extremely commonplace and are used very extensively in a wide variety of industrial, domestic, and technological applications. This gives rise to a large variation in the physical and chemical composition of abrasives as well as the shape of the abrasive. Some common uses for abrasives include grinding, polishing, buffing, honing, cutting, drilling, sharpening, lapping, and sanding (see abrasive machining). (For simplicity, "mineral" in this article will be used loosely to refer to both minerals and mineral-like substances ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen-free Copper
Oxygen-free copper (OFC) or oxygen-free high thermal conductivity (OFHC) copper is a group of wrought high-conductivity copper alloys that have been electrolytically refined to reduce the level of oxygen to 0.001% or below. Oxygen-free copper is a premium grade of copper that has a high level of conductivity and is virtually free from oxygen content. The oxygen content of copper affects its electrical properties and can reduce conductivity. Specification Oxygen-free copper is typically specified according to the ASTM/ UNS database. The UNS database includes many different compositions of high conductivity electrical copper. Of these, three are widely used and two are considered oxygen-free: * C10100 – also known as ''oxygen-free electronic'' (OFE). This is a 99.99% pure copper with 0.0005% oxygen content. It achieves a minimum 101% IACS conductivity rating. This copper is finished to a final form in a carefully regulated, oxygen-free environment. Silver (Ag) is considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deoxidation
Deoxidization is a method used in metallurgy to remove the rest of oxygen content from previously reduced iron ore during steel manufacturing. In contrast, antioxidants are used for stabilization, such as in the storage of food. Deoxidation is important in the steelmaking process as oxygen is often detrimental to the quality of steel produced. Deoxidization is mainly achieved by adding a separate chemical species to neutralize the effects of oxygen or by directly removing the oxygen. Oxidation Oxidation is the process of an element losing electrons. For example, iron will transfer two of its electrons to oxygen, forming an oxide. This occurs all throughout as an unintended part of the steelmaking process. Oxygen blowing is a method of steelmaking where oxygen is blown through pig iron to lower the carbon content. Oxygen forms oxides with the unwanted elements, such as carbon, silicon, phosphorus, and manganese, which appear from various stages of the manufacturing process. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength and low raw material cost, steel is one of the most commonly manufactured materials in the world. Steel is used in structures (as concrete Rebar, reinforcing rods), in Bridge, bridges, infrastructure, Tool, tools, Ship, ships, Train, trains, Car, cars, Bicycle, bicycles, Machine, machines, Home appliance, electrical appliances, furniture, and Weapon, weapons. Iron is always the main element in steel, but other elements are used to produce various grades of steel demonstrating altered material, mechanical, and microstructural properties. Stainless steels, for example, typically contain 18% chromium and exhibit improved corrosion and Redox, oxidation resistance versus its carbon steel counterpart. Under atmospheric pressures, steels generally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have properties that differ from those of the pure elements from which they are made. The vast majority of metals used for commercial purposes are alloyed to improve their properties or behavior, such as increased strength, hardness or corrosion resistance. Metals may also be alloyed to reduce their overall cost, for instance alloys of gold and Copper(II) sulfate, copper. A typical example of an alloy is SAE 304 stainless steel, 304 grade stainless steel which is commonly used for kitchen utensils, pans, knives and forks. Sometime also known as 18/8, it as an alloy consisting broadly of 74% iron, 18% chromium and 8% nickel. The chromium and nickel alloying elements add strength and hardness to the majority iron element, but their main function is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |