|
COVID-19 Vaccination Campaign In Italy
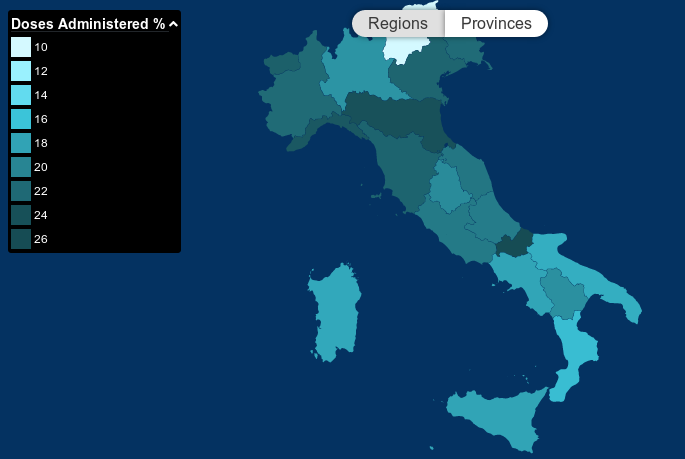
The COVID-19 vaccination campaign in Italy is a mass immunization campaign that was put in place by the Italian government in order to respond to the ongoing COVID-19 pandemic. It started on 27 December 2020, together with most countries in the European Union. The vaccination campaign was managed by the Ministry of Health and the special commissioner Domenico Arcuri until 1 March 2021, when he was replaced by Army General Francesco Paolo Figliuolo. Vaccination against COVID-19 is mandatory for all workers, older than 50, in Italy since October 15. Vaccination program In the first months of the vaccination campaign, the governmental agencies targeted the health medical and administrative personnel, together with the guests and personnel of nursing homes. In the second phase of the campaign, elderly people and public service personnel should receive the vaccine. Vaccines The Pfizer–BioNTech COVID-19 vaccine was authorized by the European Commission on 21 December 2020, on t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COVID-19 Pandemic
The COVID-19 pandemic, also known as the coronavirus pandemic, is an ongoing global pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The novel virus was first identified in an outbreak in the Chinese city of Wuhan in December 2019. Attempts to contain it there failed, allowing the virus to spread to other areas of Asia and later COVID-19 pandemic by country and territory, worldwide. The World Health Organization (WHO) declared the outbreak a public health emergency of international concern on 30 January 2020, and a pandemic on 11 March 2020. As of , the pandemic had caused COVID-19 pandemic cases, more than cases and COVID-19 pandemic deaths, confirmed deaths, making it one of the deadliest pandemics in history, deadliest in history. COVID-19 symptoms range from Asymptomatic, undetectable to deadly, but most commonly include fever, Nocturnal cough, dry cough, and fatigue. Severe illness is more likely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Johnson & Johnson
Johnson & Johnson (J&J) is an American multinational corporation founded in 1886 that develops medical devices, pharmaceuticals, and consumer packaged goods. Its common stock is a component of the Dow Jones Industrial Average and the company is ranked No. 36 on the 2021 Fortune 500 list of the largest United States corporations by total revenue. Johnson & Johnson is one of the world's most valuable companies, and is one of only two U.S.-based companies that has a prime credit rating of AAA, higher than that of the United States government. Johnson & Johnson is headquartered in New Brunswick, New Jersey, the consumer division being located in Skillman, New Jersey. The corporation includes some 250 subsidiary companies with operations in 60 countries and products sold in over 175 countries. Johnson & Johnson had worldwide sales of $93.8billion during calendar year 2021. Johnson & Johnson's brands include numerous household names of medications and first aid supplies. Among it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Campania
(man), it, Campana (woman) , population_note = , population_blank1_title = , population_blank1 = , demographics_type1 = , demographics1_footnotes = , demographics1_title1 = , demographics1_info1 = , demographics1_title2 = , demographics1_info2 = , demographics1_title3 = , demographics1_info3 = , timezone1 = CET , utc_offset1 = +1 , timezone1_DST = CEST , utc_offset1_DST = +2 , postal_code_type = , postal_code = , area_code_type = ISO 3166 code , area_code = IT-72 , blank_name_sec1 = GDP (nominal) , blank_info_sec1 = €108 billion (2018) , blank1_name_sec1 = GDP per capita , blank1_info_sec1 = €18,600 (2018) , blank2_name_sec1 = HDI (2018) , blank2_info_sec1 = 0.845 · 19th of 21 , blank_name_sec2 = NUTS Region , blank_info_sec2 = ITF , website ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
South Africa
South Africa, officially the Republic of South Africa (RSA), is the southernmost country in Africa. It is bounded to the south by of coastline that stretch along the South Atlantic and Indian Oceans; to the north by the neighbouring countries of Namibia, Botswana, and Zimbabwe; and to the east and northeast by Mozambique and Eswatini. It also completely enclaves the country Lesotho. It is the southernmost country on the mainland of the Old World, and the second-most populous country located entirely south of the equator, after Tanzania. South Africa is a biodiversity hotspot, with unique biomes, plant and animal life. With over 60 million people, the country is the world's 24th-most populous nation and covers an area of . South Africa has three capital cities, with the executive, judicial and legislative branches of government based in Pretoria, Bloemfontein, and Cape Town respectively. The largest city is Johannesburg. About 80% of the population are Black Sou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rome
, established_title = Founded , established_date = 753 BC , founder = King Romulus ( legendary) , image_map = Map of comune of Rome (metropolitan city of Capital Rome, region Lazio, Italy).svg , map_caption = The territory of the ''comune'' (''Roma Capitale'', in red) inside the Metropolitan City of Rome (''Città Metropolitana di Roma'', in yellow). The white spot in the centre is Vatican City. , pushpin_map = Italy#Europe , pushpin_map_caption = Location within Italy##Location within Europe , pushpin_relief = yes , coordinates = , coor_pinpoint = , subdivision_type = Country , subdivision_name = Italy , subdivision_type2 = Regions of Italy, Region , subdivision_name2 = Lazio , subdivision_type3 = Metropolitan cities of Italy, Metropolitan city , subdivision_name3 = Metropolitan City of Rome Capital, Rome Capital , government_footnotes= , government_type = Mayor–council gover ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spallanzani Hospital
The Lazzaro Spallanzani National Institute for Infectious Diseases (Italian: ''Istituto nazionale per le malattie infettive "L. Spallanzani"'') is an infectious disease hospital in the Italian city of Rome. The institute is named for the eighteenth-century Italian biologist Lazzaro Spallanzani. It is the Italian national reference center for Ebola patients. During the COVID-19 pandemic, the Spallanzani Institute was the first research centre in Europe to isolate the genomic sequence of SARS-CoV-2 and upload it to GenBank. The team was composed of Maria Rosaria Capobianchi, Francesca Colavita, and Concetta Castilletti. The institute is working with biotech company ReiThera to test a COVID-19 vaccine candidate called GRAd-COV2, which is based on a modified gorilla adenovirus vector Vector most often refers to: *Euclidean vector, a quantity with a magnitude and a direction *Vector (epidemiology), an agent that carries and transmits an infectious pathogen into another living or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lazio
it, Laziale , population_note = , population_blank1_title = , population_blank1 = , demographics_type1 = , demographics1_footnotes = , demographics1_title1 = , demographics1_info1 = , demographics1_title2 = , demographics1_info2 = , demographics1_title3 = , demographics1_info3 = , timezone1 = CET , utc_offset1 = +1 , timezone1_DST = CEST , utc_offset1_DST = +2 , postal_code_type = , postal_code = , area_code_type = ISO 3166 code , area_code = IT-62 , blank_name_sec1 = GDP (nominal) , blank_info_sec1 = €201 billion (2019) , blank1_name_sec1 = GDP per capita , blank1_info_sec1 = €34,300 (2019) , blank2_name_sec1 = HDI (2019) , blank2_info_sec1 = 0.914 · 3rd of 21 , blank_name_sec2 = NUTS Region , blank_info_sec2 = ITE , website w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GRAd-COV2
GRAd-COV2 is a COVID-19 vaccine candidate developed by ReiThera Srl and Lazzaro Spallanzani National Institute for Infectious Diseases The Lazzaro Spallanzani National Institute for Infectious Diseases (Italian: ''Istituto nazionale per le malattie infettive "L. Spallanzani"'') is an infectious disease hospital in the Italian city of Rome. The institute is named for the eighteenth- .... It is based on a novel replication defective Gorilla Adenovirus and encodes for SARS-COV-2 full length prefusion stabilized Spike protein. More specifically, the vector used is the simian group C adenovirus GRAd32, isolated from a captive gorilla, with a genome deleted of the entire E1 and E3 regions and the native E4 region replaced with the E4 orf6 of human adenovirus 5 (hAd5). References External links Adenoviridae Clinical trials related to COVID-19 Italian COVID-19 vaccines Science and technology in Italy Viral vector vaccines {{COVID19-vaccine-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CureVac COVID-19 Vaccine
The CureVac COVID-19 vaccine (abbreviated CVnCoV) was a COVID-19 vaccine candidate developed by CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI). The vaccine showed inadequate results in its Phase III trials with only 47% efficacy. In October 2021 CureVac abandoned further development and production plans for CVnCoV and refocused efforts on a cooperation with GlaxoSmithKline. Efficacy On 16 June 2021, CureVac said its vaccine showed 47% efficacy from its Phase IIb/III trial. Later, the final result data showed an efficacy of 48% against symptomatic disease in all age groups and, for people aged 18 to 60 years, an efficacy of 53% against symptomatic disease, 77% against moderate and severe disease and 100% against hospitalization and death, as no cases were detected in the study. This was based on interim analysis of 134 COVID cases in its Phase III study conducted in Europe and Latin America. The final analysis for the trials requires a minimum of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sanofi–GSK COVID-19 Vaccine
The Sanofi–GSK COVID-19 vaccine sold under the brand name VidPrevtyn Beta, is a COVID-19 vaccine developed by Sanofi Pasteur and GSK. The Sanofi–GSK COVID19 vaccine was approved for medical use in the European Union in November 2022. Medical uses The Sanofi–GSK COVID19 vaccine is used as a booster for active immunisation against SARSCoV2 virus in order to prevent COVID19. Pharmacology The Sanofi–GSK COVID19 vaccine is a recombinant protein subunit vaccine containing the SARS-CoV-2 spike protein, which is produced in insect cells via a baculovirus vector. It also includes an adjuvant made by GSK. It uses the same technology as Sanofi's Flublok influenza vaccine. History The Sanofi–GSK COVID19 vaccine is under development by the French pharmaceutical company Sanofi and the British-American pharmaceutical company GlaxoSmithKline. Advanced clinical trials of the vaccine were delayed in December 2020 after it failed to produce a strong immune response in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VLA2001
Valneva COVID-19 vaccine is a COVID-19 vaccine developed by French biotechnology company Valneva SE in collaboration with the American biopharmaceutical company Dynavax Technologies. In April 2022, the United Kingdom Medicines and Healthcare products Regulatory Agency (MHRA) approved the vaccine, being the first in the world to do so. It was approved for medical use in the European Union in June 2022. Technology It is a whole inactivated virus vaccine, grown in culture using the Vero cell line and inactivated with BPL. It also contains two adjuvants: alum and CpG 1018. It uses the same manufacturing technology as Valneva's Ixiaro vaccine for Japanese encephalitis. History Clinical trials Valneva COVID-19 vaccine completed phase I/II trial with 153 participants in the United Kingdom. The trials were supported by the UK National Institute for Health Research and four British universities. In April 2021, Valneva COVID-19 vaccine commenced phase III trials with approxim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Novavax COVID-19 Vaccine
The Novavax COVID-19 vaccine, sold under the brand names Nuvaxovid and Covovax, among others, is a subunit COVID-19 vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations (CEPI). Full results from Nuvaxovid's pivotal phase III trial were published in December 2021. Medical uses The Novavax COVID19 vaccine is indicated for active immunization to prevent COVID19 caused by SARS-CoV-2. Efficacy A vaccine is generally considered effective if the estimate is ≥50% with a >30% lower limit of the 95% confidence interval. Efficacy is closely related to effectiveness, which is generally expected to slowly decrease over time. In December 2021, Novavax reported, that its phase III trial showed the vaccine achieved its primary endpoint of preventing infection at least seven days after the second dose. Overall efficacy against different SARS-CoV-2s was 90.4% and efficacy against moderate-to-severe disease was 100%. Trials were conducted before the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |