|
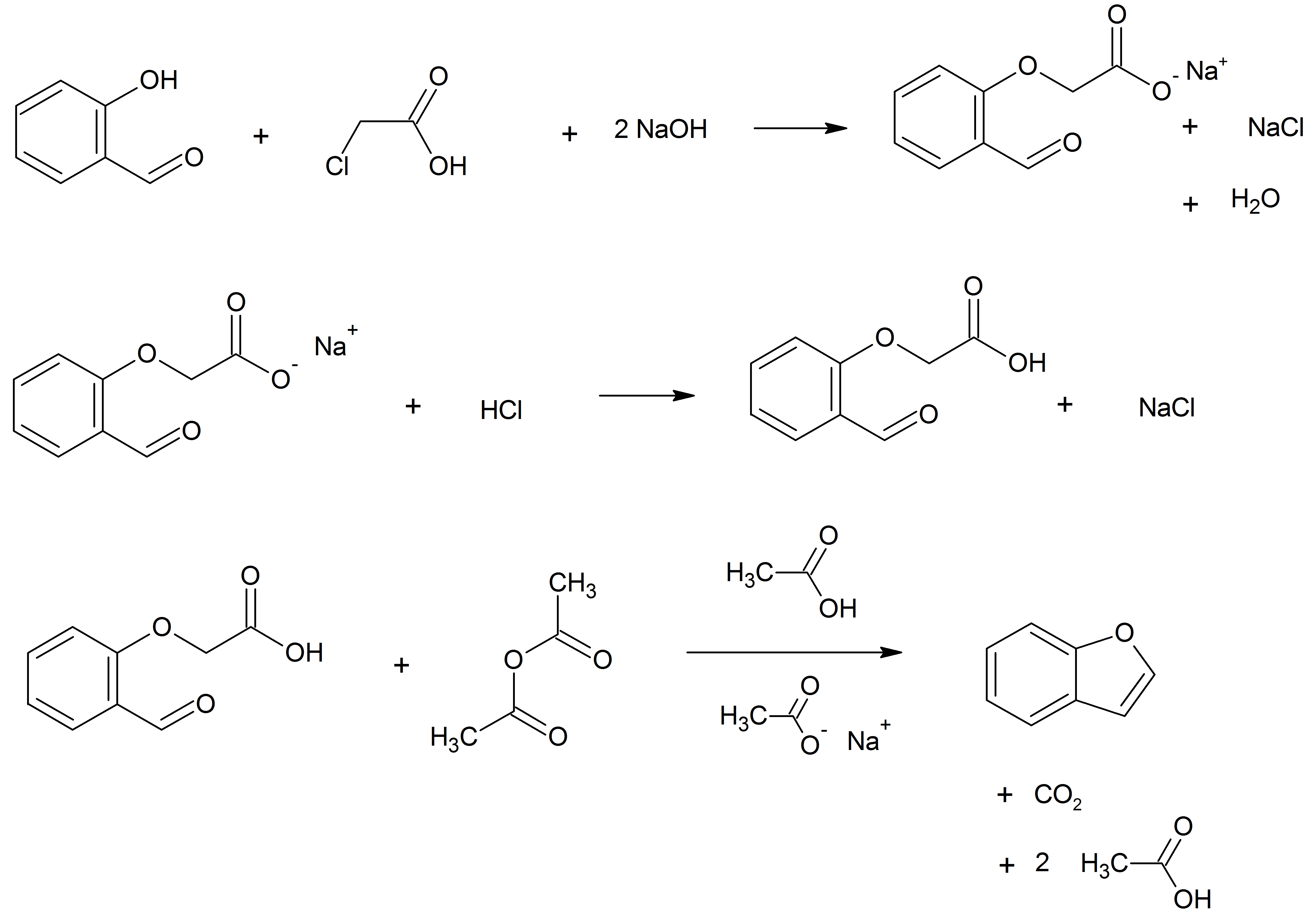
Benzofuran Ethers At The Benzene Ring
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : * Cycloisomerization of alkyne ortho-substituted phenols: : Related compounds * Substituted benzofurans * Dibenzofuran, an analog with a second fused b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perkin Rearrangement
The Perkin rearrangement (coumarin–benzofuran ring contraction) is a rearrangement reaction in which a 2-halocoumarin in the presence of hydroxide undergoes a ring contraction to form a benzofuran. The name reaction recognizes William Henry Perkin, who first reported it in 1870. Several proposals have been made for the reaction mechanism, all of which involve initial opening of the lactone to give a carboxylate and phenolate Phenolates (also called phenoxides) are anions, salt (chemistry), salts, and esters of phenols, containing the phenolate ion. They may be formed by reaction of phenols with strong base. Properties Alkali metal phenolates, such as sodium phenoxi .... : References {{organic-chem-stub Benzofurans Coumarins Name reactions Rearrangement reactions Ring contraction reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides, according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to electrophilic aromatic substitutions. Condensation with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arene Substitution Pattern
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution * In ''ortho''-substitution, two substituents occupy positions next to each other, which may be numbered 1 and 2. In the diagram, these positions are marked R and ''ortho''. * In ''meta''-substitution, the substituents occupy positions 1 and 3 (corresponding to R and ''meta'' in the diagram). * In ''para''-substitution, the substituents occupy the opposite ends (positions 1 and 4, corresponding to R and ''para'' in the diagram). The toluidines serve as an example for these three types of substitution. Synthesis Electron donating groups, for example amino, hydroxyl, alkyl, and phenyl groups tend to be ''ortho''/''para''-directors, and electron withdrawing groups such as nitro, nitrile, and ketone groups, tend to be ''meta''-directors. Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the activation energy for the isomerization reaction is sufficiently small, both isomers can often be observed and the equilibrium ratio will shift in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry to convert straight chain alkanes to isoparaffins as exemplified in the conversion of normal octane to 2,5-dimethylhexane (an "isoparaffin"): : Fuels containing branched hydrocarbons are favored for internal combustion engines for their higher octan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzofuran Synthesis Via A Diels-Alder Reaction On Vinyl Furans
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. * Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : * Cycloisomerization of alkyne ortho-substituted phenols: : Related compounds * Substituted benzofurans * Dibenzofuran, an analog with a second fused ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furans
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents, including alcohol, ether, and acetone, and is slightly soluble in water. Its odor is "strong, ethereal; chloroform-like". It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals. History The name "furan" comes from the Latin ''furfur'', which means bran (furfural is produced from bran). The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse. Furan itself was first prepared by Heinrich Limpr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroalkene
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through polar effect, alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions. Synthesis Nitroalkenes are synthesized by various means, notable examples include: * Nitroaldol reactions such as the Henry reaction: : * Nitration of an alkene with nitryl iodide generated ''in-situ'' from silver nitrite and elemental iodine: * Direct nitration of alkenes with nitric oxide and an aluminum oxide catalyst in acidic conditions: *Direct nitration of alkenes with Clayfen (Iron(III) nitrate supported on Montmorillonite clay): * Dehydration of nitro-alcohols: Reactions Nitroalkenes are useful Precursor (chemistry), intermediates for various chemical functionalities. * A nitroalkene behaving as a Michael acceptor in the synthesis of Ly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
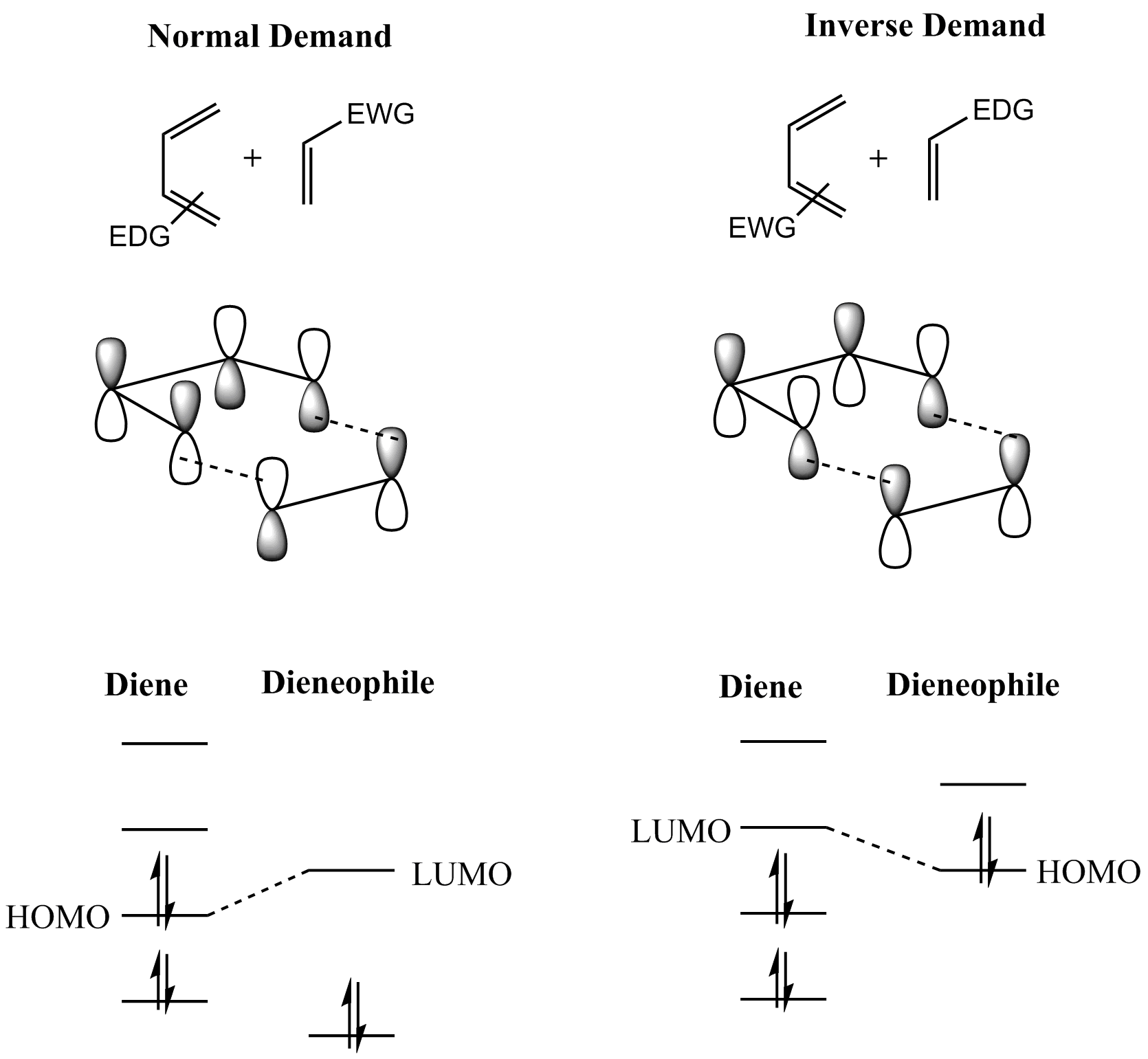
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted reaction, concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann rules, Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The Chemical Society, Perkin Transactions 2
Perkin Transactions is a scientific journal devoted to organic chemistry published from 1997 to 2002 by the Royal Society of Chemistry. It was split into ''Perkin Transactions I'' and ''Perkin Transactions II''. The predecessor journals published by the Chemical Society before the merger of that Society with other Societies to form the Royal Society of Chemistry were the ''Journal of the Chemical Society, Perkin Transactions 1'' and ''Journal of the Chemical Society, Perkin Transactions 2'' (1972-1996). They were replaced by ''Organic and Biomolecular Chemistry''. The name honours the chemist Arthur George Perkin. See also * List of scientific journals in chemistry * List of scientific journals External links * Royal Society of Chemistry'Website for Perkin 1* Royal Society of Chemistry The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |