|
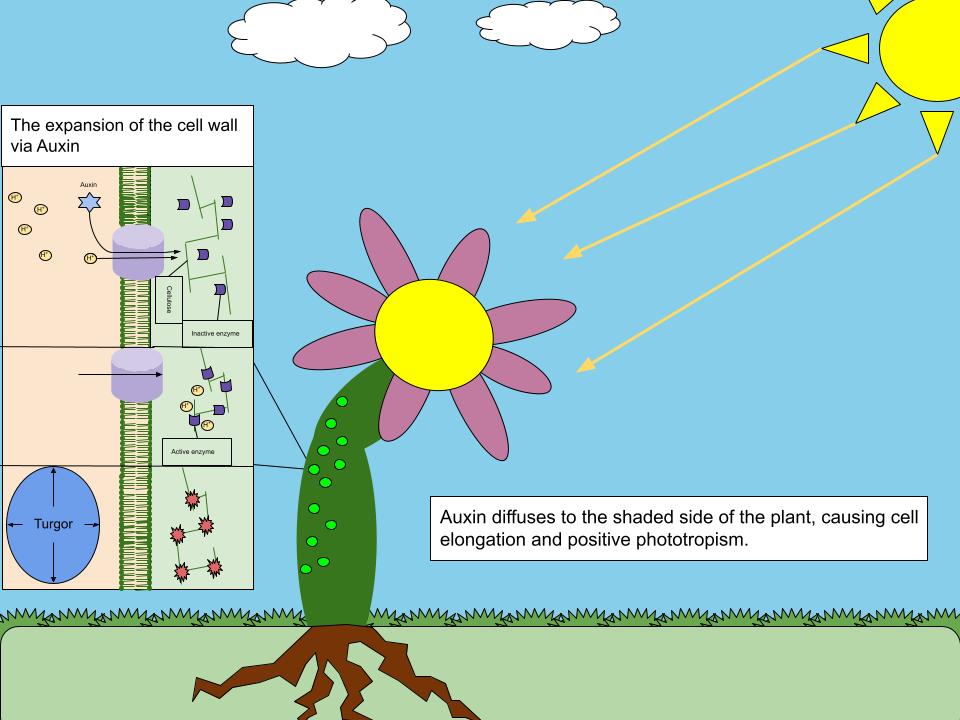
Auxin Action
Auxins (plural of auxin ) are a class of plant hormones (or plant-growth regulators) with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essential for plant body development. The Dutch biologist Frits Warmolt Went first described auxins and their role in plant growth in the 1920s. Kenneth V. Thimann became the first to isolate one of these phytohormones and to determine its chemical structure as indole-3-acetic acid (IAA). Went and Thimann co-authored a book on plant hormones, ''Phytohormones'', in 1937. Overview Auxins were the first of the major plant hormones to be discovered. They derive their name from the Greek word ( – 'to grow/increase'). Auxin is present in all parts of a plant, although in very different concentrations. The concentration in each position is crucial developmental information, so it is subject to tight regulation through both metabolism and transport ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole-3-acetic Acid
Indole-3-acetic acid (IAA, 3-IAA) is the most common naturally occurring plant hormone of the auxin class. It is the best known of the auxins, and has been the subject of extensive studies by plant physiologists. IAA is a derivative of indole, containing a carboxymethyl substituent. It is a colorless solid that is soluble in polar organic solvents. Biosynthesis IAA is predominantly produced in cells of the apex ( bud) and very young leaves of a plant. Plants can synthesize IAA by several independent biosynthetic pathways. Four of them start from tryptophan, but there is also a biosynthetic pathway independent of tryptophan. Plants mainly produce IAA from tryptophan through indole-3-pyruvic acid. IAA is also produced from tryptophan through indole-3-acetaldoxime in ''Arabidopsis thaliana''. In rats, IAA is a product of both endogenous and colonic microbial metabolism from dietary tryptophan along with tryptophol. This was first observed in rats infected by '' Trypanosoma br ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polar Auxin Transport
Polar auxin transport is the regulated transport of the plant hormone auxin in plants. It is an active process, the hormone is transported in cell-to-cell manner and one of the main features of the transport is its asymmetry and directionality ( polarity). The polar auxin transport functions to coordinate plant development; the following spatial auxin distribution underpins most of plant growth responses to its environment and plant growth and developmental changes in general. In other words, the flow and relative concentrations of auxin informs each plant cell where it is located and therefore what it should do or become. Chemiosmotic model Polar auxin transport (PAT) is directional and active flow of auxin molecules through the plant tissues. The flow of auxin molecules through the neighboring cells is driven by carriers (''type of membrane transport protein'') in the cell-to-cell fashion (from one cell to other cell and then to the next one) and the direction of the flow i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenoxyacetic Acid
Phenoxyacetic acid, POA, is a white solid with the formula of C8H8O3. Although not itself usefully active as an herbicide, it forms the part-structure of many phenoxy herbicide derivatives including MCPA and 2,4-D. Structure and synthesis Phenoxyacetic acid is an ''O''-phenyl derivative of glycolic acid. It is both a monocarboxylic acid and an aryl ether. Its preparation from sodium phenolate and sodium chloroacetate in hot water was first reported in 1880. :1) C6H5O−Na+ + ClCH2COO−Na+ → C6H5OCH2COO−Na+ + NaCl :2) C6H5OCH2COO−Na+ + HCl → C6H5OCH2COOH + NaCl The phenolate anion reacts via nucleophilic attack on the methylene carbon of the chloroacetic acid, forming an ether bond. Properties Phenoxyacetic acid is a white or clear crystalline compound at room temperature. When impure, it can appear to be a light tan to brown. The compound has a solubility in water of 12 g/L and is highly soluble in organic solvents including ethanol, diethyl ether and be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clopyralid
Clopyralid (3,6-dichloro-2-pyridinecarboxylic acid) is a selective herbicide used for control of broadleaf weeds, especially thistles and clovers. Clopyralid is in the picolinic acid family of herbicides, which also includes aminopyralid, picloram, triclopyr, and several less common herbicides.Staff, Virginia Tech Cooperative Extension. Revised May 14, 201Pyridine Herbicide Carryover: Causes and Precautions Accessed May 27, 2013 For control of creeping thistle, '' Cirsium arvense'', a noxious, perennial weed, clopyralid is one of the few effective herbicides available. It is particularly damaging to peas, tomatoes, and sunflowers, and can render potatoes, lettuce, and spinach inedible. It does not affect grasses (family Poaceae). Clopyralid is known for its ability to persist in dead plants and compost, and has accumulated to phytotoxic levels in finished compost in a few highly publicized cases. This first came to light in Washington, when during 2000 and 2001, residues of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triclopyr
Triclopyr (3,5,6-trichloro-2-pyridinyloxyacetic acid) is an organic compound in the pyridine group that is used as a systemic foliar herbicide and fungicide. History Triclopyr triethylammonium (TEA) was first registered in 1979 in the United States for use as an herbicide in non-crop areas and forestry to control broadleaf weeds and woody plants. A year later, in 1980, a formulation containing triclopyr butoxyethyl ester (BEE) was registered for similar applications. Both formulations expanded their usage to turf sites in 1984, and in 1985, triclopyr BEE was specifically registered for use on rangeland and permanent grass pastures. In 1995, the triclopyr TEA formulation received registration for use on rice crops to manage broadleaf weed species. Uses Triclopyr is a systemic herbicide and is selectively used to control dicotyledonous (broadleaf) weeds and woody plants while leaving monocotyledonous plants (such as grasses, bulbs, and conifers) largely unaffected. It is clas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picloram
Picloram is a systemic herbicide used for general woody plant control. It also controls a wide range of broad-leaved weeds, but most grasses are resistant. Pesticide Management Education Program, . A chlorinated derivative of picolinic acid, picloram is in the family of herbicides. Picloram can be sprayed on foliage, injected into plants, applied to cut surfaces, or placed at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridinecarboxylic Acid
A pyridinecarboxylic acid is any member of a group of Organic compound, organic compounds which are Carboxy group, monocarboxylic Derivative (chemistry), derivatives of pyridine. Pyridinecarboxylic acid comes in three Isomer, isomers: *Picolinic acid (2-pyridinecarboxylic acid) *Nicotinic acid (3-pyridinecarboxylic acid), also known as Niacin (substance), Niacin *Isonicotinic acid (4-pyridinecarboxylic acid) All isomers share the molecular weight 123,11 g/mol and the chemical formula C6H5NO2. See also * Pyridinedicarboxylic acid {{chemistry index Pyridines Aromatic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinclorac
Quinclorac is an organic compound with the formula . A colorless solid, it is soluble in hydrocarbons and alcohols. The compound is the carboxylic acid of 3,7-dichloroquinoline. Applications Quinclorac is an herbicide used primarily to control crabgrass. It is found in some household herbicides for lawn use. Most lawn maintenance companies use the product for the control of annual grass weeds like crabgrass. Quinclorac is a synthetic auxin. Heap considers it to also have a cellulose herbicide action, although some studies show quinclorac to have no cellulose action. Regulation and registration Quinclorac is not approved to use in the European Union due to toxicity concerns. Resistance Resistance to quinclorac is of concern in soybean cultivation. In rice, Graminaceous resistance is produced by the cytochrome Cytochromes are redox-active proteins containing a heme, with a central iron (Fe) atom at its core, as a cofactor. They are involved in the electron transport cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
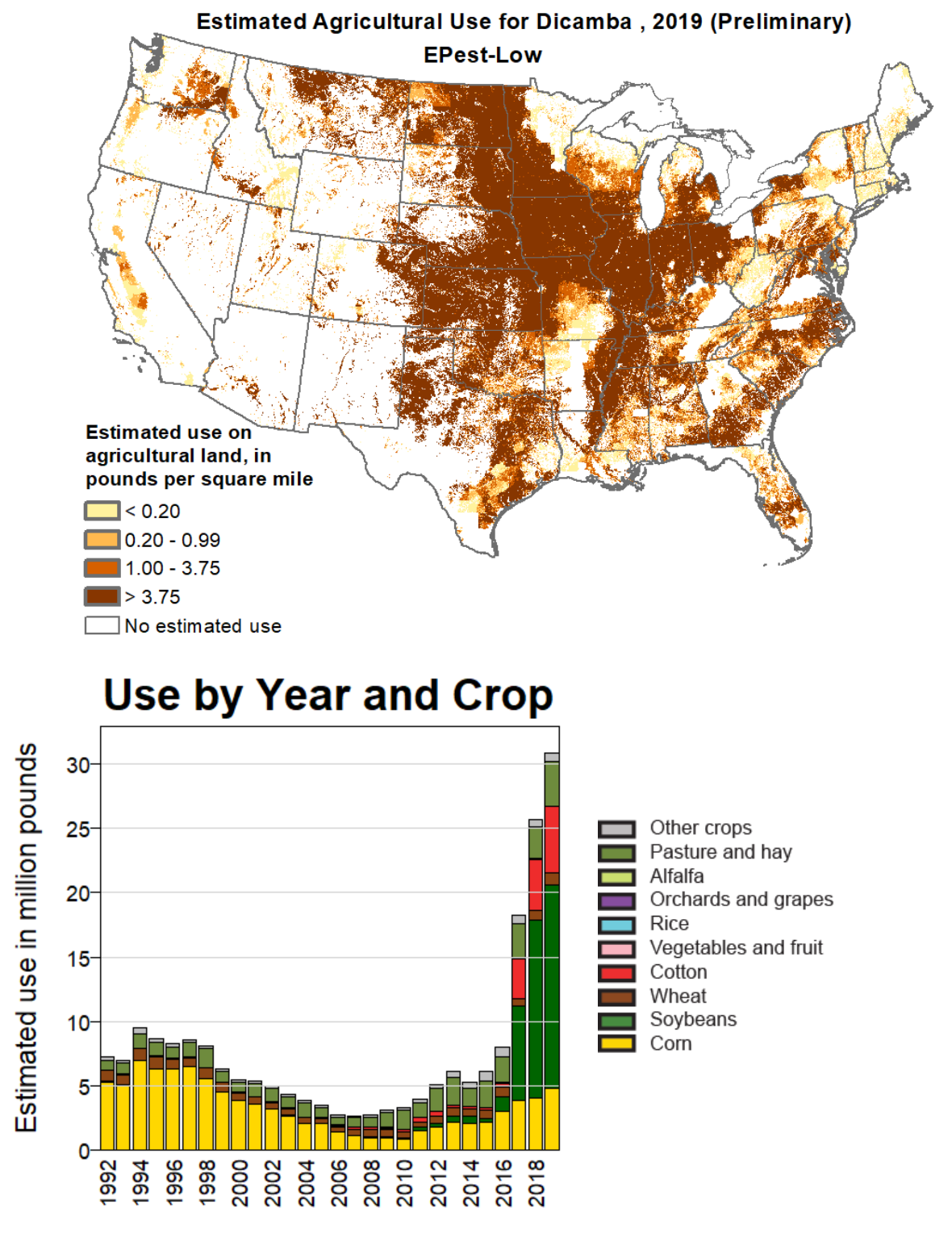
Dicamba
Dicamba (3,6-dichloro-2-methoxybenzoic acid) is a selective systemic herbicide first registered in 1967. Brand names for formulations of this herbicide include Dianat, Banvel, Diablo, Oracle and Vanquish. This chemical compound is a chlorinated derivative of ''o''-anisic acid. It has been described as a "widely used, low-cost, environmentally friendly herbicide that does not persist in soils and shows little or no toxicity to wildlife and humans." Despite its success in improving crop yields, dicamba has attracted controversy. According to the United States Environmental Protection Agency (EPA), dicamba's primary ecological risk is for non-target terrestrial plants from exposure through spray drift, whereby dicamba inadvertently migrates to non-targeted neighboring areas, damaging those plants. In 2016, dicamba was approved for use in the United States over GMO dicamba-resistant crops created by Monsanto. Dicamba came under significant scrutiny due to its tendency to sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole-3-propionic Acid
3-Indolepropionic acid (IPA), or indole-3-propionic acid, has been studied for its therapeutic value in the treatment of Alzheimer's disease. As of 2022 IPA shows potential in the treatment of this disease, though the therapeutic effect of IPA depends on dose and time of therapy initiation. Though promising in some historical clinical trials, IPA is not clinically listed as a useful therapeutic in managing Alzheimer's as of 2023. IPA is an even more potent scavenger of hydroxyl radicals than melatonin, the most potent scavenger of hydroxyl radicals that is synthesized by human enzymes. Similar to melatonin but unlike other antioxidants, it scavenges radicals without subsequently generating reactive and pro-oxidant intermediate compounds. Occurrence Biosynthesis in humans and cellular effects This compound is endogenously produced by human microbiota and has only been detected ''in vivo'' when the species '' Clostridium sporogenes'' is present in the gastrointestinal tract. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole-3-butyric Acid
Indole-3-butyric acid (1''H''-indole-3-butanoic acid, IBA) is a white to light-yellow crystalline solid, with the molecular formula C12H13NO2. It melts at 125°C in atmospheric pressure and decomposes before boiling. IBA is a plant hormone in the auxin family and is an ingredient in many commercial horticultural plant rooting products. Plant hormone Since IBA is not completely soluble in water, it is typically dissolved in 75% aqueous ethanol (aqua vitae) for use in plant rooting, making a solution of between 10,000 and 50,000 ppm. This alcohol solution is then diluted with distilled water to the desired concentration. IBA is also available as a alkali metal salt, which is soluble in water. The solution should be kept in a cool, dark place for best results. This compound had been thought to be strictly synthetic; however, it was reported that the compound was isolated from leaves and seeds of maize and other species. In maize, IBA has been shown to be biosynthesized in vi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugation (biochemistry)
Bioconjugation is a chemical strategy to form a stable Covalent bond, covalent link between two molecules, at least one of which is a biomolecule. Methods to conjugate biomolecules are applied in various field, including medicine, diagnostics, biocatalysis and materials. Synthetically modified biomolecules can have diverse functionalities, such as tracking cellular events, revealing enzyme function, determining protein biodistribution, Imaging science, imaging specific biomarkers, and delivering Pharmaceutical drug, drugs to targeted cells. Bioconjugation is a crucial strategy that links these modified biomolecules with different Substrate (biochemistry), substrates. Besides applications in biomedical research, bioconjugation has recently also gained importance in nanotechnology such as bioconjugated quantum dots. The most common types of bioconjugation include coupling of a small molecule (such as biotin or a fluorescent dye) to a protein. Antibody-drug conjugates such as Br ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |