|
Allotropes Of Carbon
Carbon is capable of forming many allotropy, allotropes (structurally different forms of the same element) due to its Valence (chemistry), valency (Tetravalence, tetravalent). Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include carbon nano tube, nanotubes, Carbon nanobud, nanobuds and Graphene nanoribbon, nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA). Atomic and diatomic carbon Under certain conditions, carbon can be found in its atomic form. It can be formed by vaporizing graphite, by passing large electric currents to form a carbon arc under very low pressure. It is extrem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eight Allotropes Of Carbon
8 (eight) is the natural number following 7 and preceding 9. Etymology English ''eight'', from Old English '', æhta'', Proto-Germanic ''*ahto'' is a direct continuation of Proto-Indo-European '' *oḱtṓ(w)-'', and as such cognate with Greek and Latin , both of which stems are reflected by the English prefix oct(o)-, as in the ordinal adjective ''octaval'' or ''octavary'', the distributive adjective is ''octonary''. The adjective ''octuple'' (Latin ) may also be used as a noun, meaning "a set of eight items"; the diminutive ''octuplet'' is mostly used to refer to eight siblings delivered in one birth. The Semitic numeral is based on a root ''*θmn-'', whence Akkadian ''smn-'', Arabic ''ṯmn-'', Hebrew ''šmn-'' etc. The Chinese numeral, written (Mandarin: ''bā''; Cantonese: ''baat''), is from Old Chinese ''*priāt-'', ultimately from Sino-Tibetan ''b-r-gyat'' or ''b-g-ryat'' which also yielded Tibetan '' brgyat''. It has been argued that, as the cardinal num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Comet
A comet is an icy, small Solar System body that warms and begins to release gases when passing close to the Sun, a process called outgassing. This produces an extended, gravitationally unbound atmosphere or Coma (cometary), coma surrounding the nucleus, and sometimes a Comet tail, tail of gas and dust gas blown out from the coma. These phenomena are due to the effects of solar radiation and the outstreaming solar wind plasma acting upon the nucleus of the comet. Comet nuclei range from a few hundred meters to tens of kilometers across and are composed of loose collections of ice, dust, and small rocky particles. The coma may be up to 15 times Earth's diameter, while the tail may stretch beyond one astronomical unit. If sufficiently close and bright, a comet may be seen from Earth without the aid of a telescope and can Subtended angle, subtend an arc of up to 30° (60 Moons) across the sky. Comets have been observed and recorded since ancient times by many cultures and religion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Angle
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of a molecule, i.e. they can be understood as approximately local and hence transferable properties. Determination The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography, neutron diffraction and electron diffraction can give molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane Conformation
Cyclohexane conformations are any of several three-dimensional shapes adopted by cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are important prototypes of a wide range of compounds. The internal angles of a regular, flat hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle (the arc cosine of −). Therefore, the cyclohexane ring tends to assume non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. Consider the carbon atoms numbered from 1 to 6 around the ring. If we hold carbon atoms 1, 2, and 3 stationary, with the correct bond lengths and the tetrahedral angle between the two bonds, and then continue by adding carbon atoms 4, 5, and 6 with the correct bond length and the tetrahedral angle, we can vary the three dihedral angles f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccos(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group ''Td'', but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram at left, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge len ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term "covalence" was introduced by Irving Langmuir in 1919, with Nevil Sidgwick using "co-valent link" in the 1920s. Merriam-Webster dates the specific phrase ''covalent bond'' to 1939, recognizing its first known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond Cubic
In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, including α-tin, the semiconductors silicon and germanium, and silicon–germanium alloys in any proportion. There are also crystals, such as the high-temperature form of cristobalite, which have a similar structure, with one kind of atom (such as silicon in cristobalite) at the positions of carbon atoms in diamond but with another kind of atom (such as oxygen) halfway between those (see :Minerals in space group 227). Although often called the diamond lattice, this structure is not a lattice in the technical sense of this word used in mathematics. Crystallographic structure Diamond's cubic structure is in the Fdm space group (space group 227), which follows the face-centered cubic Bravais lattice. The lattice describes the repeat pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Face-centred Cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc) Note: the term fcc is often used in synonym for the ''cubic close-packed'' or ccp structure occurring in metals. However, fcc stands for a face-centered cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed. Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais latices ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The Geology, geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock (geology), rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate (geology), aggregate of two or more different types of minerals, spaci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
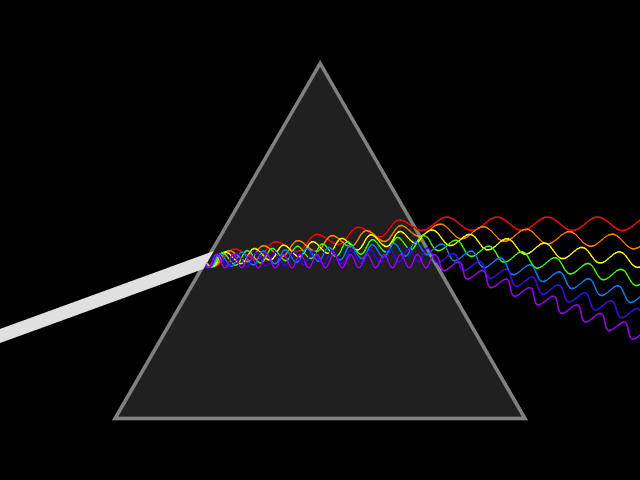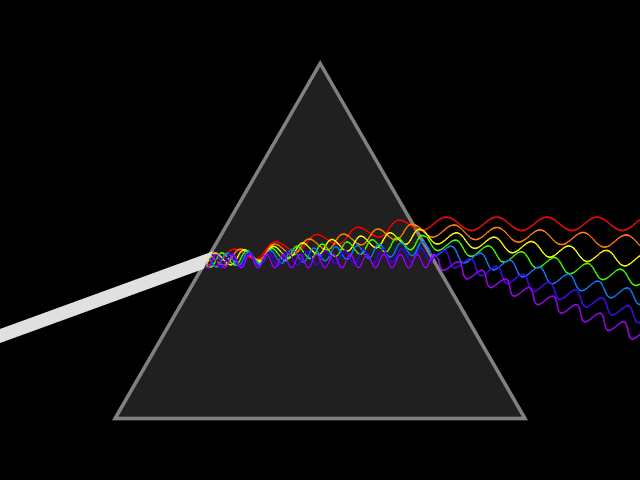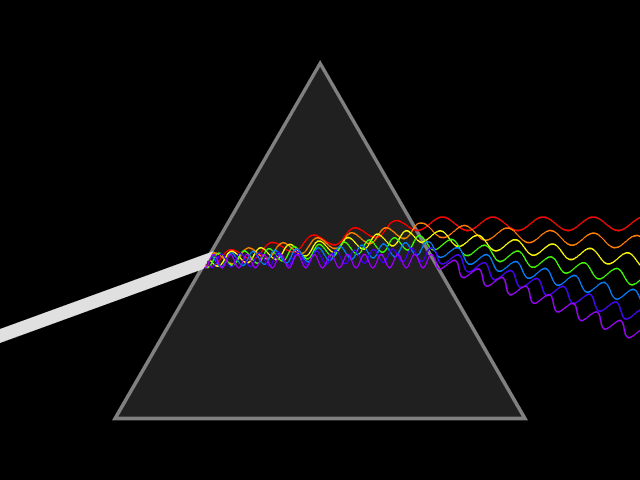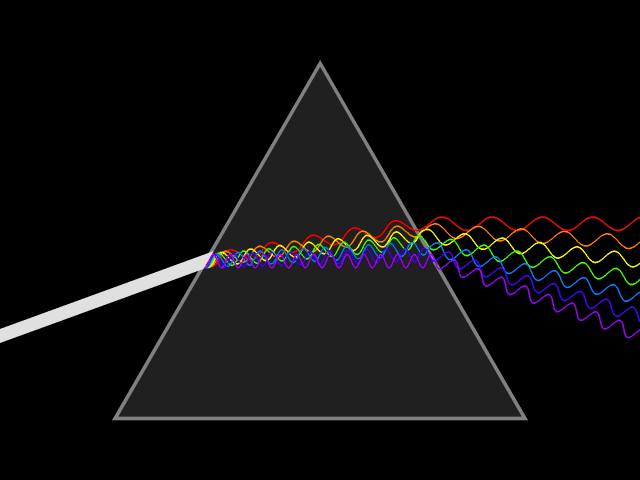
Dispersion (optics)
Dispersion is the phenomenon in which the phase velocity of a wave depends on its frequency. Sometimes the term chromatic dispersion is used to refer to optics specifically, as opposed to wave propagation in general. A medium having this common property may be termed a dispersive medium. Although the term is used in the field of optics to describe light and other electromagnetic waves, dispersion in the same sense can apply to any sort of wave motion such as acoustic dispersion in the case of sound and seismic waves, and in gravity waves (ocean waves). Within optics, dispersion is a property of telecommunication signals along transmission lines (such as microwaves in coaxial cable) or the Pulse (signal processing), pulses of light in optical fiber. In optics, one important and familiar consequence of dispersion is the change in the angle of refraction of different colors of light, as seen in the spectrum produced by a dispersive Prism (optics), prism and in chromatic aberration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refractive Index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refraction, refracted, when entering a material. This is described by Snell's law of refraction, , where and are the angle of incidence (optics), angle of incidence and angle of refraction, respectively, of a ray crossing the interface between two media with refractive indices and . The refractive indices also determine the amount of light that is reflectivity, reflected when reaching the interface, as well as the critical angle for total internal reflection, their intensity (Fresnel equations) and Brewster's angle. The refractive index, n, can be seen as the factor by which the speed and the wavelength of the radiation are reduced with respect to their vacuum values: the speed of light in a medium is , and similarly the wavelength in that me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |