|
ATPase Inhibitors
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, ATP hydrolase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life. Some such enzymes are integral membrane proteins (anchored within biological membranes), and move solutes across the membrane, typically against their concentration gradient. These are called transmembrane ATPases. Functions Transmembrane ATPases import metabolites necessary for cell metabolism and export toxins, wastes, and solutes that can hinder cellular processes. An important example is the sodium-potassium pump (Na+/K+ATPase) that maintains the cell membrane potential. Another example is the hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (biology)
The cell is the basic structural and functional unit of all life, forms of life. Every cell consists of cytoplasm enclosed within a Cell membrane, membrane; many cells contain organelles, each with a specific function. The term comes from the Latin word meaning 'small room'. Most cells are only visible under a light microscope, microscope. Cells Abiogenesis, emerged on Earth about 4 billion years ago. All cells are capable of Self-replication, replication, protein synthesis, and cell motility, motility. Cells are broadly categorized into two types: eukaryotic cells, which possess a Cell nucleus, nucleus, and prokaryotic, prokaryotic cells, which lack a nucleus but have a nucleoid region. Prokaryotes are single-celled organisms such as bacteria, whereas eukaryotes can be either single-celled, such as amoebae, or multicellular organism, multicellular, such as some algae, plants, animals, and fungi. Eukaryotic cells contain organelles including Mitochondrion, mitochondria, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active Transport
In cellular biology, active transport is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate (ATP), and secondary active transport that uses an electrochemical gradient. This process is in contrast to passive transport, which allows molecules or ions to move down their concentration gradient, from an area of high concentration to an area of low concentration, with energy. Active transport is essential for various physiological processes, such as nutrient uptake, hormone secretion, and nig impulse transmission. For example, the sodium-potassium pump uses ATP to pump sodium ions out of the cell and potassium ions into the cell, maintaining a concentration gradient essential for cellular function. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Design
Protein design is the rational design of new protein molecules to design novel activity, behavior, or purpose, and to advance basic understanding of protein function. Proteins can be designed from scratch (''de novo'' design) or by making calculated variants of a known protein structure and its sequence (termed ''protein redesign''). Rational protein design approaches make protein-sequence predictions that will fold to specific structures. These predicted sequences can then be validated experimentally through methods such as peptide synthesis, site-directed mutagenesis, or artificial gene synthesis. Rational protein design dates back to the mid-1970s. Recently, however, there were numerous examples of successful rational design of water-soluble and even transmembrane peptides and proteins, in part due to a better understanding of different factors contributing to protein structure stability and development of better computational methods. Overview and history The goal in ratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nest (protein Structural Motif)
The Nest is a type of Structural motif, protein structural motif. It is a small recurring anion-binding feature of both proteins and peptides. Each consists of the main chain atoms of three consecutive amino acid residues. The main chain NH groups bind the anions while the side chain atoms are often not involved. Proline residues lack NH groups so are rare in nests. About one in 12 of amino acid residues in proteins, on average, belongs to a nest. Nest conformations The conformation of a nest is such that the NH groups of the first and third amino acid residues are liable to be hydrogen bonded to a negatively charged, or partially negatively charged, atom, often an oxygen atom. The NH of the second residue may also be hydrogen bonded to the same atom but usually points somewhat away. These main chain atoms form a concavity called a nest into which an anionic atom fits. Such anionic atoms are sometimes called eggs and more than one egg may occur bound to a nest. The oxyanion ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix). The alpha helix is the most common structural arrangement in the Protein secondary structure, secondary structure of proteins. It is also the most extreme type of local structure, and it is the local structure that is most easily predicted from a sequence of amino acids. The alpha helix has a right-handed helix conformation in which every backbone amino, N−H group hydrogen bonds to the backbone carbonyl, C=O group of the amino acid that is four residue (biochemistry), residues earlier in the protein sequence. Other names The alpha helix is also commonly called a: * Pauling–Corey–Branson α-helix (from the names of three scientists who described its structure) * 3.613-helix because there are 3.6 amino acids in one ring, with 13 atoms being involved in the ring formed by the hydrogen bond (starting with amidic hydrogen and ending with carbonyl oxygen) Discovery ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, Alzheimer's disease and other proteinopathies. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond was planar. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
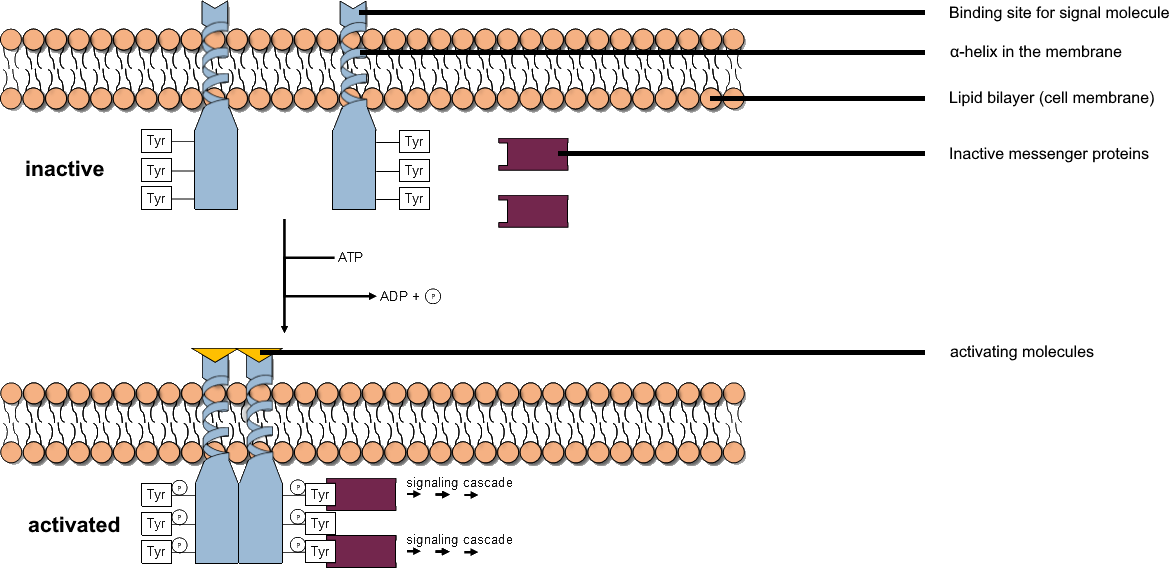
Tyrosine Kinase
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions. Tyrosine kinases belong to a larger class of enzymes known as protein kinases which also attach phosphates to other amino acids such as serine and threonine. Phosphorylation of proteins by kinases is an important mechanism for communicating signals within a cell (signal transduction) and regulating cellular activity, such as cell division. Protein kinases can become mutated, stuck in the "on" position, and cause unregulated growth of the cell, which is a necessary step for the development of cancer. Therefore, kinase inhibitors, such as imatinib and osimertinib, are often effective cancer treatments. Most tyrosine kinases have an associated protein tyrosine phosphatase, which removes the phosphate group. Reaction Protein kinases are a group of enzymes that possess a ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Walker Motifs
The Walker A and Walker B motifs are protein sequence motifs, known to have highly conserved three-dimensional structures. These were first reported in ATP-binding proteins by Walker and co-workers in 1982. Of the two motifs, the A motif is the main "P-loop" responsible for binding phosphate, while the B motif is a much less conserved downstream region. The P-loop is best known for its presence in ATP- and GTP-binding proteins, and is also found in a variety of proteins with phosphorylated substrates. Major lineages include: * RecA and rotor ATP synthase / ATPases (α and β subunits). * Nucleic acid-dependent ATPases: helicases, Swi2, and PhoH () * AAA proteins * STAND NTPases including MJ, PH, AP, and NACHT ATPases * ABC- PilT ATPases * Nucleotide kinases () * G domain proteins: G-proteins (transducin), myosin. Walker A motif Walker A motif, also known as the Walker loop, or P-loop, or phosphate-binding loop, is a motif in proteins that is associated with phospha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Co-transport
In cellular biology, active transport is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate (ATP), and secondary active transport that uses an electrochemical gradient. This process is in contrast to passive transport, which allows molecules or ions to move down their concentration gradient, from an area of high concentration to an area of low concentration, with energy. Active transport is essential for various physiological processes, such as nutrient uptake, hormone secretion, and nig impulse transmission. For example, the sodium-potassium pump uses ATP to pump sodium ions out of the cell and potassium ions into the cell, maintaining a concentration gradient essential for cellular function. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardenolides
A cardenolide is a type of steroid. Many plants contain derivatives, collectively known as cardenolides, including many in the form of cardenolide glycosides (cardenolides that contain structural groups derived from sugars). Cardenolide glycosides are often toxic; specifically, they are heart-arresting. Cardenolides are toxic to animals through inhibition of the enzyme Na+/K+-ATPase, which is responsible for maintaining the sodium and potassium ion gradients across the cell membranes. Etymology The term derives from ''card-'' "heart" (from Greek καρδία ''kardiā'') and the suffix , referring to the lactone ring with double bond at C17. Cardenolides are a class of steroids (or aglycones if viewed as cardiac glycoside constituents), and cardenolides are a subtype of this class (see MeSH D codes list). Structure Cardenolides are C(23)-steroids with methyl groups at C-10 and C-13 and a five-membered lactone (specifically a butenolide) at C-17. They are aglycone constituents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Potassium ATPase
Gastric hydrogen potassium ATPase, also known as H+/K+ ATPase, is an enzyme which functions to acidify the stomach. It is a member of the P-type ATPases, also known as E1-E2 ATPases due to its two states. Biological function and location The gastric hydrogen potassium ATPase or H+/K+ ATPase is the proton pump of the stomach. It exchanges potassium from the intestinal Lumen (anatomy), lumen with cytoplasmic hydronium and is the enzyme primarily responsible for the acidification of the stomach contents and the activation of the digestive enzyme pepsin (see gastric acid). The H+/K+ ATPase is found in parietal cells, which are highly specialized Epithelium, epithelial cells located in the inner cell lining of the stomach called the gastric mucosa. Parietal cells possess an extensive secretory membrane system and the H+/K+ ATPase is the major protein constituent of these membranes. A small amount of H+/K+ ATPase is also found in the renal medulla. Genes and protein structure Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |