|
1939 In Science
The year 1939 in science and technology involved some significant events, listed below. Astronomy * Robert Oppenheimer jointly predicts two new types of celestial object: ** With George Volkoff, he calculates the structure of neutron stars. ** With Hartland Snyder, he predicts the existence of what will come to be called black holes. Biology * Autumn – DDT's properties as an insecticide are discovered by Paul Hermann Müller, Paul Müller of Novartis#History, Geigy. Cartography * Kavrayskiy VII projection devised by Vladimir V. Kavrayskiy. Chemistry * January 7 – French physicist Marguerite Perey identifies francium, the Timeline of chemical element discoveries, last chemical element first discovered in nature, as a decay product of 227Ac. * April 30 – Nylon fabric is first introduced to the general public at the 1939 New York World's Fair, New York World's Fair. * July – Edward Adelbert Doisy of Saint Louis University publishes the chemical structure of vitamin K. * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edward Adelbert Doisy
Edward Adelbert Doisy (November 13, 1893 – October 23, 1986) was an American biochemist. He received the Nobel Prize in Physiology or Medicine in 1943 with Henrik Dam for their discovery of vitamin K (K from "Koagulations-Vitamin" in German) and its chemical structure. Doisy was born in Hume, Illinois, on November 13, 1893. He completed his A.B. degree in 1914 and his M.S. degree in 1916 from the University of Illinois at Urbana-Champaign. He completed his Ph.D. in 1920 from Harvard University. In 1919 he accepted a faculty appointment in the Department of Biochemistry at Washington University School of Medicine, where he rose in rank to associate professor. In 1923, he moved to Saint Louis University as professor and chairman of the new Department of Biochemistry. He served as professor and chairman of that department until he retired in 1965. Saint Louis University renamed the department the E.A. Doisy Department of Biochemistry, in his honor. More recently, the department ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
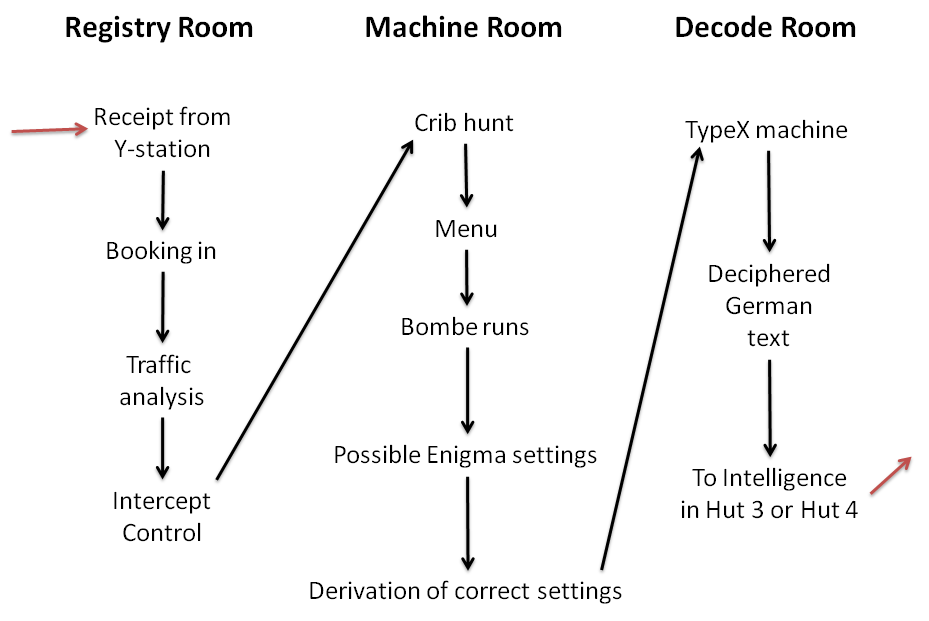
Bletchley Park
Bletchley Park is an English country house and Bletchley Park estate, estate in Bletchley, Milton Keynes (Buckinghamshire), that became the principal centre of Allies of World War II, Allied World War II cryptography, code-breaking during the Second World War. During World War II, the estate housed the Government Code and Cypher School (GC&CS), which regularly penetrated the secret communications of the Axis Powers most importantly the German Enigma machine, Enigma and Lorenz cipher, Lorenz ciphers. The GC&CS team of codebreakers included John Tiltman, Dilwyn Knox, Alan Turing, Harry Golombek, Gordon Welchman, Conel Hugh O'Donel Alexander, Hugh Alexander, Donald Michie, W. T. Tutte, Bill Tutte and Stuart Milner-Barry. The team at Bletchley Park devised automatic machinery to help with decryption, culminating in the development of Colossus computer, Colossus, the world's first programmable digital electronic computer. Codebreaking operations at Bletchley Park ended in 1946 and al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Government Code And Cypher School
The Government Code and Cypher School (GC&CS) was a British signals intelligence agency set up in 1919. During the First World War, the British Army and Royal Navy had separate signals intelligence agencies, MI1b and NID25 (initially known as Room 40) respectively. It was particularly known for its work on codebreaking at Bletchley Park and after the war became the Government Communications Headquarters (GCHQ). Interwar period In 1919, the Cabinet's Secret Service Committee, chaired by Lord Curzon, recommended that a peacetime codebreaking agency should be created, a task which was given to the Director of Naval Intelligence, Hugh Sinclair.Johnson, 1997, p. 44 Sinclair merged staff from NID25 and MI1b into the new organisation, which initially consisted of around 25–30 officers and a similar number of clerical staff. It was titled the "Government Code and Cypher School" (GC&CS), a cover-name which was chosen by Victor Forbes of the Foreign Office. Alastair Denniston, who h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gordon Welchman
William Gordon Welchman OBE (15 June 1906 – 8 October 1985) was an English mathematician. During World War II, he worked at Britain's secret decryption centre at Bletchley Park, where he was one of the most important contributors. In 1948, after the war, he moved to the US and later worked on the design of military communications systems. Early life, education and career Gordon Welchman was born, the youngest of three children, at Fishponds in Bristol, to William Welchman (1866–1954) and Elizabeth Marshall Griffith. William was a Church of England priest who had been a missionary overseas before returning to England as a country vicar, eventually becoming archdeacon of Bristol. Elizabeth was the daughter of another priest, the Revd Edward Moule Griffith. Welchman was educated at Marlborough College and then studied mathematics at Trinity College, Cambridge, from 1925 to 1928. In 1929, he became a Research Fellow in Mathematics at Sidney Sussex College, Cambridge. He be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alan Turing
Alan Mathison Turing (; 23 June 1912 – 7 June 1954) was an English mathematician, computer scientist, logician, cryptanalyst, philosopher and theoretical biologist. He was highly influential in the development of theoretical computer science, providing a formalisation of the concepts of algorithm and computation with the Turing machine, which can be considered a model of a general-purpose computer. Turing is widely considered to be the father of theoretical computer science. Born in London, Turing was raised in southern England. He graduated from University of Cambridge, King's College, Cambridge, and in 1938, earned a doctorate degree from Princeton University. During World War II, Turing worked for the Government Code and Cypher School at Bletchley Park, Britain's codebreaking centre that produced Ultra (cryptography), Ultra intelligence. He led Hut 8, the section responsible for German naval cryptanalysis. Turing devised techniques for speeding the breaking of Germ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing Chemical bond, bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. The resonance hybrid is the accurate structure for a molecule or ion; it is an average of the theoretical (or hypothetical) contributing structures. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is suffi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Bonding
Ionic bonding is a type of chemical bonding that involves the Coulomb's law, electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding, along with covalent bonding and metallic bonding. Ions are atoms (or groups of atoms) with an electrostatic charge. Atoms that gain electrons make negatively charged ions (called anions). Atoms that lose electrons make positively charged ions (called cations). This transfer of electrons is known as electrovalence in contrast to covalent bond, covalence. In the simplest case, the cation is a metal atom and the anion is a Nonmetal (chemistry), nonmetal atom, but these ions can be more complex, e.g. polyatomic ions, polyatomic ions like or . In simpler words, an ionic bond results from the transfer of electrons from a metal to a non-metal to obtain a full valence shell for both atoms. '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bonding
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term "covalence" was introduced by Irving Langmuir in 1919, with Nevil Sidgwick using "co-valent link" in the 1920s. Merriam-Webster dates the specific phrase ''covalent bond'' to 1939, recognizing its first known u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a Tetrahedral molecular geometry, tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed ou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bonding
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described as having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference" stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optimal distance (t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |