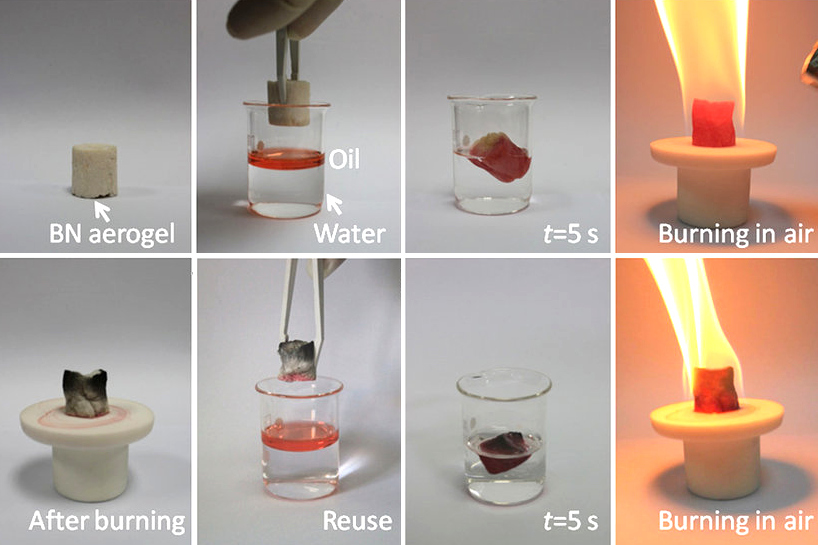
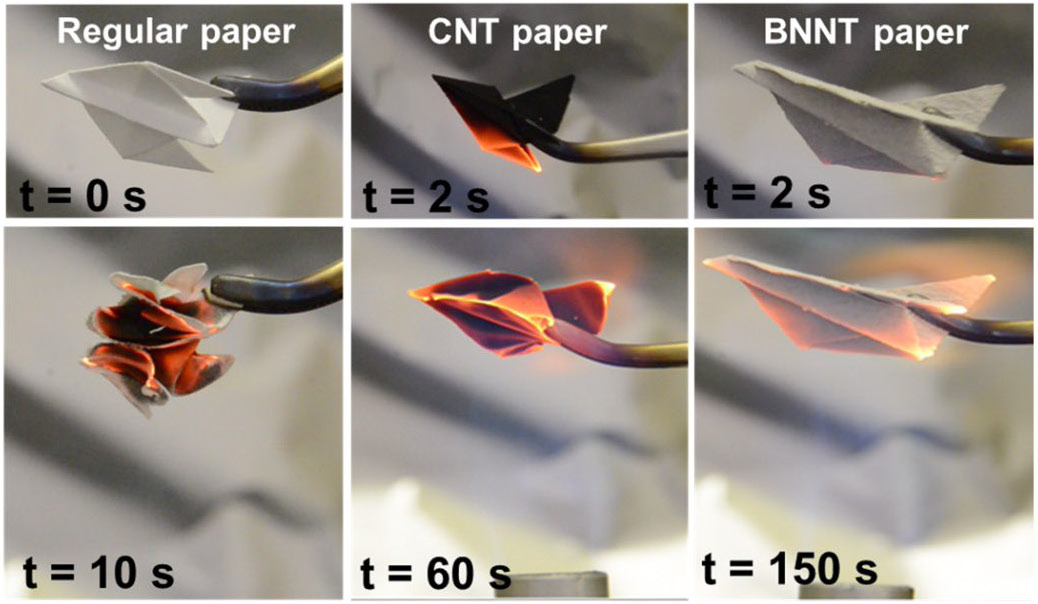
|
Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula B N. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. History Boron nitride was discovered by chemistry teacher of the Liverpool Institute in 1842 via reduction of boric acid with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal Crystal System
In crystallography, the hexagonal crystal family is one of the six crystal family, crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section hexagonal crystal family#Crystal systems, crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zincblende (crystal Structure)
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc) Note: the term fcc is often used in synonym for the ''cubic close-packed'' or ccp structure occurring in metals. However, fcc stands for a face-centered cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed. Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais latices ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula B N. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. History Boron nitride was discovered by chemistry teacher of the Liverpool Institute in 1842 via reduction of boric acid with c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tunnel Diode
A tunnel diode or Esaki diode is a type of semiconductor diode that has effectively " negative resistance" due to the quantum mechanical effect called tunneling. It was invented in August 1957 by Leo Esaki and Yuriko Kurose when working at Tokyo Tsushin Kogyo, now known as Sony. In the first public report of the discovery (presentation at the 12th annual meeting of the Physical Society of Japan in October 1957), Takashi Suzuki, who was a student at Tokyo University of Science and doing his internship at Tokyo Tsushin Kogyo under Esaki's supervision, was a co-author. Suzuki, along with Yuriko Kurose, first observed the negative differential resistance when they were testing heavily doped P-N junctions. In 1973, Esaki received the Nobel Prize in Physics for experimental demonstration of the electron tunneling effect in semiconductors. Robert Noyce independently devised the idea of a tunnel diode while working for William Shockley, but was discouraged from pursuing it. Tun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating the presence of double bonds within the carbon structure. Graphene is known for its exceptionally high Ultimate tensile strength, tensile strength, Electrical resistivity and conductivity, electrical conductivity, Transparency and translucency, transparency, and being the thinnest two-dimensional material in the world. Despite the nearly transparent nature of a single graphene sheet, graphite (formed from stacked layers of graphene) appears black because it absorbs all visible light wavelengths. On a microscopic scale, graphene is the strongest material ever measured. The existence of graphene was first theorized in 1947 by P. R. Wallace, Philip R. Wallace during his research on graphite's electronic properties, while the term ''graphen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Force
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the van der ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term "covalence" was introduced by Irving Langmuir in 1919, with Nevil Sidgwick using "co-valent link" in the 1920s. Merriam-Webster dates the specific phrase ''covalent bond'' to 1939, recognizing its first known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amorphous Carbon
Amorphous carbon is free, reactive carbon that has no crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amorphous carbon is often abbreviated to ''aC'' for general amorphous carbon, ''aC:H'' or ''HAC'' for hydrogenated amorphous carbon, or to ''ta-C'' for tetrahedral amorphous carbon (also called diamond-like carbon). In mineralogy In mineralogy, amorphous carbon is the name used for coal, carbide-derived carbon, and other impure forms of carbon that are neither graphite nor diamond. In a crystallographic sense, however, the materials are not truly amorphous but rather polycrystalline materials of graphite or diamond within an amorphous carbon matrix. Commercial carbon also usually contains significant quantities of other elements, which may also form crystalline impurities. In modern science With the development of modern thin f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Cyanide
Potassium cyanide is a compound with the formula KCN. It is a colorless salt, similar in appearance to sugar, that is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewellery for chemical gilding and buffing. Potassium cyanide is highly toxic, and a dose of 200 to 300 milligrams will kill nearly any human. The moist solid emits small amounts of hydrogen cyanide due to hydrolysis (reaction with water). Hydrogen cyanide is often described as having an odor resembling that of bitter almonds. The taste of potassium cyanide has been described as acrid and bitter, with a burning sensation similar to lye. Potassium cyanide kills so rapidly its taste hasn't been reliably documented, in 2006 an Indian man named M.P. Prasad committed suicide using potassium cyanide. He was a goldsmith and was aware of the mystery behind its taste. In the suicide note Prasad left, the final words written were that potassium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boric Acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salt (chemistry), salts, and can react with Alcohol (chemistry), alcohols to form borate esters. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds. The term "boric acid" is also used generically for any oxyacid of boron, such as metaboric acid and tetraboric acid . History Orthoboric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name ("sedative salt of Homberg"). However, boric acid and borates have been used since the time of the ancient Greece, anc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liverpool Institute
The Liverpool Institute High School for Boys was an all-boys grammar school in the English port city of Liverpool. The school had its origins in 1825 but occupied different premises while the money was found to build a dedicated building on Mount Street. The institute was first known as the Liverpool Mechanics' School of Arts. In 1832 the name was shortened to the Liverpool Mechanics' Institution. The façade of the listed building, the entrance hall and modified school hall remain after substantial internal reconstruction was completed in the early 1990s. School history in brief Its initial primary purpose as a mechanics' institute (one of many established about this time throughout the country) was to provide educational opportunities, mainly through evening classes, for working men. Lectures for the general public were also provided of wide interest covering topics ranging from Arctic exploration to Shakespeare and philosophy. Luminaries like Charles Dickens, Anthony Trollo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Casting (metalworking)
In metalworking and jewelry making, casting is a process in which a liquid metal is delivered into a Mold (manufacturing), mold (usually by a crucible) that contains a negative impression (i.e., a three-dimensional negative image) of the intended shape. The metal is poured into the mold through a hollow channel called a Sprue (manufacturing), sprue. The metal and mold are then cooled, and the metal part (the ''casting'') is extracted. Casting is most often used for making complex shapes that would be difficult or uneconomical to make by other methods. Casting processes have been known for thousands of years, and have been widely used for sculpture (especially in bronze), jewelry in precious metals, and weapons and tools. Highly engineered castings are found in 90 percent of durable goods, including cars, trucks, aerospace, trains, mining and construction equipment, oil wells, appliances, pipes, hydrants, wind turbines, nuclear plants, medical devices, defense products, toys, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |