|
B-cell Receptor
The B-cell receptor (BCR) is a transmembrane protein on the surface of a B cell. A B-cell receptor is composed of a membrane-bound immunoglobulin molecule and a signal transduction moiety. The former forms a type 1 Transmembrane protein, transmembrane receptor protein, and is typically located on the Cell membrane, outer surface of these lymphocyte cells. Through biochemical signaling and by physically acquiring antigens from the immune synapses, the BCR controls the activation of the B cell. B cells are able to gather and grab antigens by engaging biochemical modules for receptor clustering, cell spreading, generation of pulling forces, and receptor transport, which eventually culminates in endocytosis and antigen presentation. B cells' mechanical activity adheres to a pattern of negative and positive feedbacks that regulate the quantity of removed antigen by manipulating the dynamic of BCR–antigen bonds directly. Particularly, grouping and spreading increase the relation of anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Memory B Cell
In immunology, a memory B cell (MBC) is a type of B lymphocyte that forms part of the adaptive immune system. These cells develop within germinal centers of the secondary lymphoid organs. Memory B cells circulate in the blood stream in a quiescent state, sometimes for decades. Their function is to memorize the characteristics of the antigen that activated their parent B cell during initial infection such that if the memory B cell later encounters the same antigen, it triggers an accelerated and robust secondary immune response. Memory B cells have B cell receptors (BCRs) on their cell membrane, identical to the one on their parent cell, that allow them to recognize antigen and mount a specific antibody response. Development and activation T cell dependent mechanisms In a T-cell dependent development pathway, naïve follicular B cells are activated by antigen-presenting follicular B helper T cells (TFH) during the initial infection, or primary immune response. Naïve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterodimers
In biochemistry, a protein dimer is a macromolecular complex or multimer formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins while a protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and hetero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin M
Immunoglobulin M (IgM) is the largest of several isotypes of antibodies (also known as immunoglobulin) that are produced by vertebrates. IgM is the first antibody to appear in the response to initial exposure to an antigen; causing it to also be called an acute phase antibody. In humans and other mammals that have been studied, plasmablasts in the spleen are the main source of specific IgM production. History In 1937, an antibody was observed in horses hyper-immunized with pneumococcus polysaccharide that was much larger in size than the typical rabbit γ-globulin, with a molecular weight of 990,000 daltons. In accordance with its larger size, the new antibody was originally referred to as γ-macroglobulin, and subsequently termed IgM—M for “macro”. The V domains of normal immunoglobulin are highly heterogeneous, reflecting their role in protecting against the great variety of infectious microbes, and this heterogeneity impeded detailed structural analysis of IgM. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fragment Antigen-binding
The fragment antigen-binding region (Fab region) is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope (the antigen-binding site), comprising a set of complementarity-determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen. Preparation In an experimental setting, Fc and Fab fragments can be generated in the laboratory. The enzyme papain can be used to cleave an immunoglobulin monomer into two Fab fragments and an Fc fragment. Conversely, the enzyme pepsin cleaves below the hinge region, so the result instead is a F(ab')2 fragment and a pFc' fragment. Recently another enzyme for generation of F(ab')2 has been commercially available. The enzyme IdeS (Immunoglobulin degrading enzyme from ''Streptococcus pyogenes'', trade name FabRICATOR) cleaves IgG in a sequence specific manner a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoreceptor Tyrosine-based Activation Motif
An immunoreceptor tyrosine-based activation motif (ITAM) is a conserved sequence of four amino acids that is repeated twice in the cytoplasmic tails of non-catalytic tyrosine-phosphorylated receptors, cell-surface proteins found mainly on immune cells. Its major role is being an integral component for the initiation of a variety of signaling pathway and subsequently the activation of immune cells, although different functions have been described, for example an osteoclast maturation. Structure The motif contains a tyrosine separated from a leucine or isoleucine by any two other amino acids, giving the signature YxxL/I. Two of these signatures are typically separated by between 6 and 8 amino acids in the cytoplasmic tail of the molecule (YxxL/Ix(6-8)YxxL/I). However, in various sources, this consensus sequence differs, mainly in the number of amino acids between individual signatures. Apart from ITAMs which have the structure described above, there is also a variety of proteins c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are the cytosol (a gel-like substance), the cell's internal sub-structures, and various cytoplasmic inclusions. In eukaryotes the cytoplasm also includes the nucleus, and other membrane-bound organelles.The cytoplasm is about 80% water and is usually colorless. The submicroscopic ground cell substance, or cytoplasmic matrix, that remains after the exclusion of the cell organelles and particles is groundplasm. It is the hyaloplasm of light microscopy, a highly complex, polyphasic system in which all resolvable cytoplasmic elements are suspended, including the larger organelles such as the ribosomes, mitochondria, plant plasti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide Bond
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups. In inorganic chemistry, the anion appears in a few rare minerals, but the functional group has tremendous importance in biochemistry. Disulfide bridges formed between thiol groups in two cysteine residues are an important component of the tertiary and quaternary structure of proteins. Compounds of the form are usually called '' persulfides'' instead. Organic disulfides Structure Disulfides have a C–S–S–C dihedral angle approaching 90°. The S–S bond length is 2.03 Å in diphenyl disulfide, similar to that in elemental sulfur. Disulfides are usually symmetric but they can also be unsymmetric. Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organosulfur chemistry are symmetrical disulfides. Unsy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
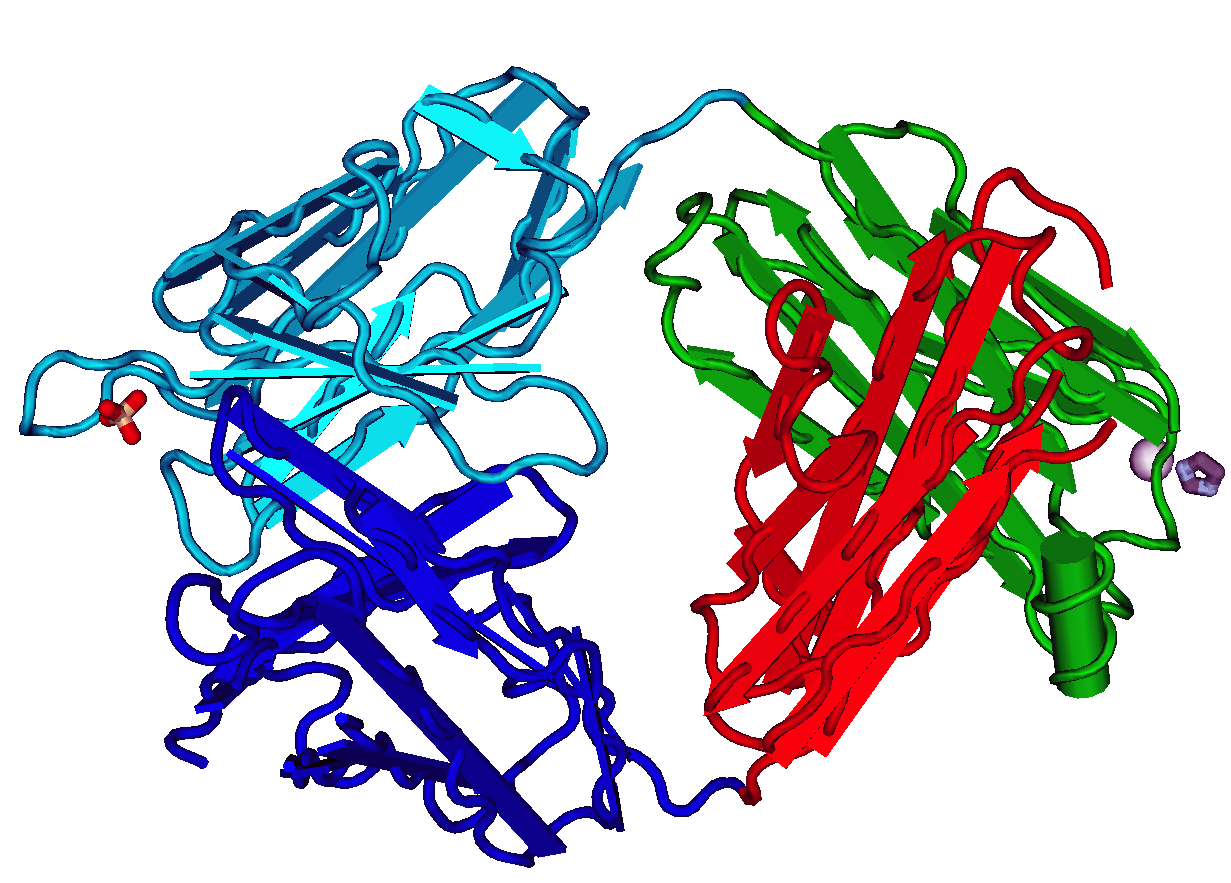
CD79
Introduction CD79 ( Cluster of Differentiation 79) is a transmembrane protein that forms a complex with the B-cell receptor (BCR) and generates a signal following recognition of antigen by the BCR. CD79 is composed of two distinct chains called CD79A and CD79B (also known as Igα and Igβ); these form a heterodimer on the surface of a B cell stabilized by disulfide bonding. CD79a and CD79b are both members of the immunoglobulin superfamily. Human CD79a is encoded by the ''mb-1'' gene that is located on chromosome 19, and CD79b is encoded by the ''B29'' gene that located on chromosome 17. Both CD79 chains contain an immunoreceptor tyrosine-based activation motif (ITAM) in their intracellular tails that they use to propagate a signal in a B cell, in a similar manner to CD3-generated signal transduction observed during T cell receptor activation on T cells. Function CD79 serves to be a pan-B cell marker for the detection of B-cell neoplasms. However, tumor cells in some case ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterodimer
In biochemistry, a protein dimer is a macromolecular complex or multimer formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins while a protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and hetero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Alpha-helix
A transmembrane domain (TMD, TM domain) is a membrane-spanning protein domain. TMDs may consist of one or several alpha-helices or a transmembrane beta barrel. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels can contain polar residues. TMDs vary greatly in size and hydrophobicity; they may adopt organelle-specific properties. Functions of transmembrane domains Transmembrane domains are known to perform a variety of functions. These include: * Anchoring transmembrane proteins to the membrane. *Facilitating molecular transport of molecules such as ions and proteins across biological membranes; usually hydrophilic residues and binding sites in the TMDs help in this process. *Signal transduction across the membrane; many transmembrane proteins, such as G protein-coupled receptors, receive extracellular signals. TMDs then propagate those signals across the mem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Figure 42 02 06
Figure may refer to: General *A shape, drawing, depiction, or geometric configuration *Figure (wood), wood appearance *Figure (music), distinguished from musical motif *Noise figure, in telecommunication *Dance figure, an elementary dance pattern *A person's figure, human physical appearance *Figure–ground (perception), the distinction between a visually perceived object and its surroundings Arts *Figurine, a miniature statuette representation of a creature *Action figure, a posable jointed solid plastic character figurine *Figure painting, realistic representation, especially of the human form *Figure drawing *Model figure, a scale model of a creature Writing *figure, in writing, a type of floating block (text, table, or graphic separate from the main text) *Figure of speech, also called a rhetorical figure *Christ figure, a type of character * in typesetting, text figures and lining figures Accounting *Figure, a synonym for number *Significant figures in a decimal number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |