|
Anion Exchange Membrane Electrolysis
Anion exchange membrane (AEM) electrolysis is the electrolysis of water that utilises a semipermeable membrane that conducts hydroxide ions (OH−) called an anion exchange membrane. Like a proton-exchange membrane (PEM), the membrane separates the products, provides electrical insulation between electrodes, and conducts ions. Unlike PEM, AEM conducts hydroxide ions. AEM electrolysis is still in the early research and development stage, while alkaline water electrolysis is mature and PEM electrolysis is in the commercial stage. There is less academic literature on pure-water fed AEM electrolysers compared to the usage of KOH solution. One advantage of AEM water electrolysis is that a high-cost noble metal catalyst is not required, low-cost transition metal catalyst can be used instead. AEM electrolysis is similar to alkaline water electrolysis, which uses a non-ion-selective separator instead of an anion-exchange membrane. __TOC__ Advantages and challenges Advantages Of all w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AEM Water Electrolysis Working Principle With HER And OER
AEM or A.E.M. may refer to: Aviation * Aviation Electrician's Mate, a rating of the U.S. Navy * IATA airport code for Amgu Airport in Primorsky Krai, Russia Companies, groups, organizations * (English: ''Mexican Space Agency''), the national space agency of Mexico * Agnico Eagle Mines Limited, a Canadian-based gold producer * Association of Equipment Manufacturers, a United States-based trade association * Societé d'Application Electro-Mécanique (AEM), manufacturer of the AEM (1924 automobile) Engineering, science and technology * AEM rubber, an ethylene acrylic rubber * Alkaline earth metal, the 6 chemical elements in group 2 of the periodic table. * α-Ethylmescaline, a psychedelic drug * Analytic element method, a numerical technique * Anion exchange membrane, a type of semipermeable membrane used in fuel cells * Applications Explorer Mission, part of the Explorer program * ''Applied and Environmental Microbiology,'' a scientific research journal * Applied element met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen Evolution Reaction
Oxygen is a chemical element; it has symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will bind covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula . Dioxygen gas currently constitutes approximately 20.95% molar fraction of the Earth's atmosphere, though this has changed considerably over long periods of time in Earth's history. A much rarer triatom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Timeline Of Hydrogen Technologies
This is a timeline of the history of hydrogen technology. Timeline 16th century * c. 1520 – First recorded observation of hydrogen by Paracelsus through dissolution of metals (iron, zinc, and tin) in sulfuric acid. 17th century * 1625 – First description of hydrogen by Jan Baptist van Helmont, Johann Baptista van Helmont. First to use the word "gas". * 1650 – Theodore de Mayerne, Turquet de Mayerne obtains a gas or "inflammable air" by the action of dilute sulphuric acid on iron. * 1662 – Boyle's law (gas law relating pressure and volume). * 1670 – Robert Boyle produces hydrogen by reacting metals with acid. * 1672 – "New Experiments touching the Relation between Flame and Air" by Robert Boyle. * 1679 – Denis Papin – safety valve. * 1700 – Nicolas Lemery shows that the gas produced in the sulfuric acid/iron reaction is explosive in air. 18th century * 1755 – Joseph Black confirms that different gases exist. / Latent heat * 1766 – Henry Cavendish publishes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photocatalytic Water Splitting
Photocatalytic water splitting is a process that uses photocatalysis for the dissociation of water (H2O) into hydrogen () and oxygen (). The inputs are light energy (photons), water, and a catalyst(s). The process is inspired by Photosynthesis, which converts water and carbon dioxide into oxygen and carbohydrates. Water splitting using solar radiation has not been commercialized. Photocatalytic water splitting is done by dispersing photocatalyst particles in water or depositing them on a substrate, unlike Photoelectrochemical cell, which are assembled into a cell with a photoelectrode. Hydrogen fuel production using water and light (photocatalytic water splitting), instead of petroleum, is an important renewable energy strategy. Concepts Two mole of is split into 1 mole and 2 mole using light in the process shown below. : \begin\\ \text \ce\\ \text \ce\\ \text \ce\\ \text \ce\\ \end A photon with an energy greater than 1.23 eV is needed to generate an el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
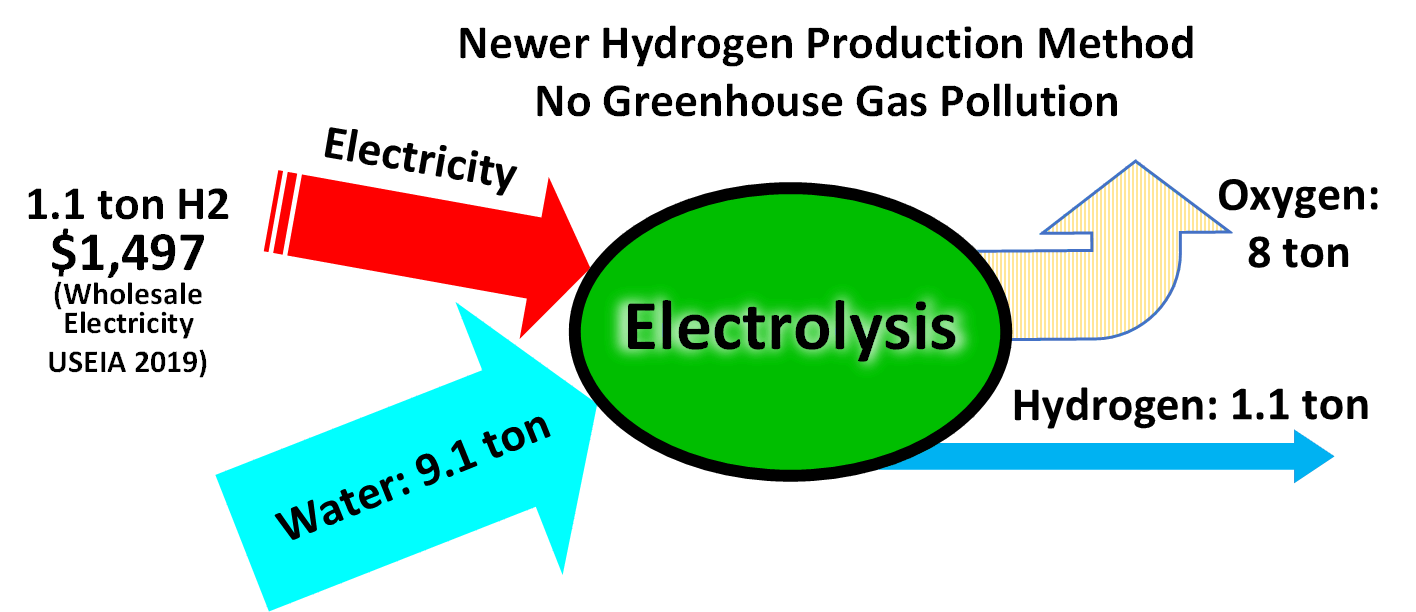
Hydrogen Production
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Article in press. Most hydrogen is ''gray hydrogen'' made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as ''blue hydrogen''. ''Green hydrogen'' is usually understood to be produced from Renewable energy, renewable electricity via electrolysis of water. Less frequently, definitions of ''green hydrogen'' include hydrogen produced from other low-emission sources such as Biomass (energy), biomass. Producing green hydrogen is currently more expensive than producing gray hydrogen, and the efficiency of energy conversion is inherently low. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of chemical element, elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity." Etymology The word "electrolysis" was introduced by Michael Faraday in 1834, using the Greek language, Greek words "amber", which since the 17th century was associated with electrical phenomena, and ' meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure chemical element, elements, precedes the coinage of the term and formal description by Faraday. History In the early nineteenth century, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Engineering
Electrochemical engineering is the branch of chemical engineering dealing with the technological applications of electrochemical phenomena, such as electrosynthesis of chemicals, electrowinning and refining of metals, flow batteries and fuel cells, surface modification by electrodeposition, electrochemical separations and corrosion. According to the IUPAC, the term ''electrochemical engineering'' is reserved for electricity-intensive processes for industrial or energy storage applications and should not be confused with ''applied electrochemistry'', which comprises small batteries, amperometric sensors, microfluidic devices, microelectrodes, solid-state devices, voltammetry at disc electrodes, etc. More than 6% of the electricity is consumed by large-scale electrochemical operations in the US. Scope Electrochemical engineering combines the study of heterogeneous charge transfer at electrode/electrolyte interphases with the development of practical materials and proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronically conducting phase (typically an external electrical circuit, but not necessarily, as in Electroless nickel-phosphorus plating, electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic chemical species, species in a Solution (chemistry), solution). When a chemical reaction is driven by an electrical Voltage, potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the aforementioned electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionomer
An ionomer () ('' iono-'' + '' -mer'') is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole percent are ionized. The ionized units are often carboxylic acid groups. The classification of a polymer as an ionomer depends on the level of substitution of ionic groups as well as how the ionic groups are incorporated into the polymer structure. For example, polyelectrolytes also have ionic groups covalently bonded to the polymer backbone, but have a much higher ionic group molar substitution level (usually greater than 80%); ionenes are polymers where ionic groups are part of the actual polymer backbone. These two classes of ionic-group-containing polymers have vastly different morphological and physical properties and are therefore not considered ionomers. Ionomers have unique physical properties including electrical conductivity and v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Electrode Assembly
A membrane electrode assembly (MEA) is an assembled stack of proton-exchange membranes (PEM) or alkali anion exchange membrane (AAEM), catalyst and flat plate electrode used in fuel cells and polymer electrolyte membrane electrolysis, electrolyzers. PEM-MEA The PEM is sandwiched between two electrodes which have the catalyst embedded in them. The electrodes are electrically insulated from each other by the PEM. These two electrodes make up the anode and cathode respectively. The PEM is typically a fluoropolymer (PFSA) proton permeable Electrical insulation, electrical insulator barrier. Hydrocarbon variants are currently being developed and are expected to succeed fluoropolymers. This barrier allows the transport of the protons from the anode to the cathode through the membrane but forces the electrons to travel around a conductive path to the cathode. The most commonly used Nafion PEMs are Nafion XL, 112, 115, 117, and 1110. The electrodes are heat pressed onto the PEM. Commonly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SN2 Reaction
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted (i.e. simultaneous) fashion. The name SN2 refers to the Hughes-Ingold symbol of the mechanism: "SN" indicates that the reaction is a nucleophilic substitution, and "2" that it proceeds via a bimolecular mechanism, which means both the reacting species are involved in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic substitution, the SN1 reaction, is that the displacement of the leaving group, which is the rate-determining step, is separate from the nucleophilic attack in SN1. The SN2 reaction can be considered as an organic-chemistry analogue of the associative substitution from the field of inorganic chemistry. Reaction mech ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hofmann Degradation
The Hofmann rearrangement (Hofmann degradation) is the organic reaction of a primary amide to a primary amine with one less carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines. The reaction is named after its discoverer, August Wilhelm von Hofmann, and should not be confused with the Hofmann elimination, another name reaction for which he is eponymous. Mechanism The reaction of bromine with sodium hydroxide forms sodium hypobromite ''in situ'', which transforms the primary amide into an intermediate isocyanate. The formation of an intermediate nitrene is not possible because it implies also the formation of a hydroxamic acid as a byproduct, which has never been observed. The intermediate isocyanate is hydrolyzed to a primary amine, giving off carbon dioxide. #Base abstracts an acidic N-H proton, yi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |