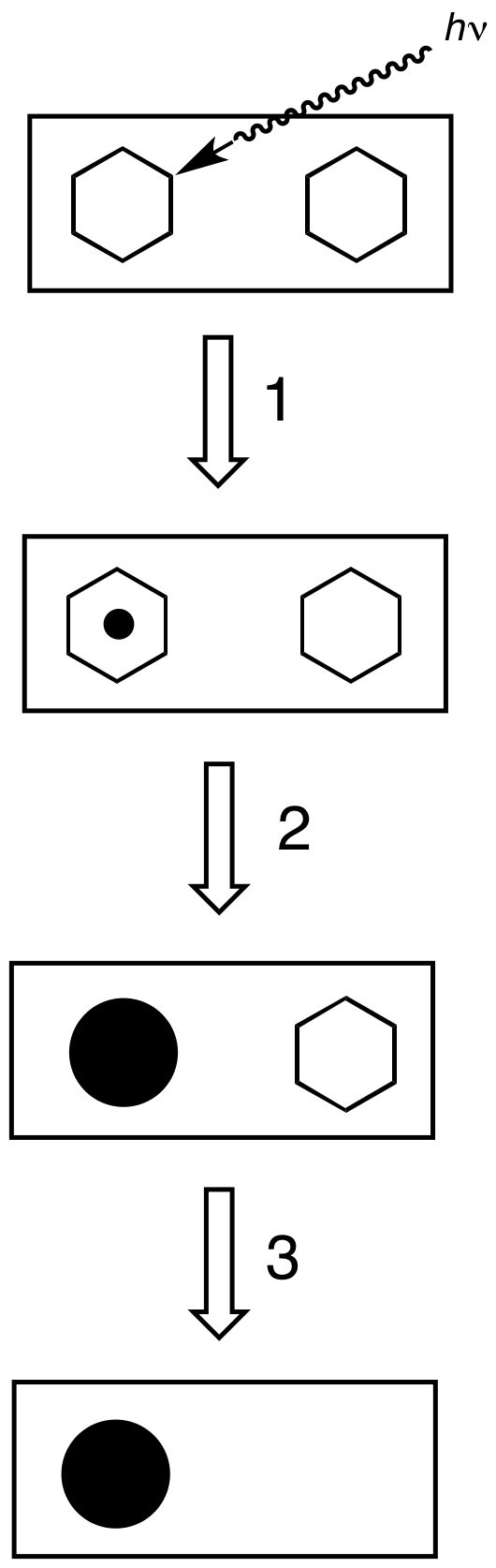
|
Ammonium Hydrosulfide
Ammonium hydrosulfide is the chemical compound with the formula . Composition It is the salt derived from the ammonium cation and the hydrosulfide anion. The salt exists as colourless, water-soluble, micaceous crystals. On Earth the compound is encountered mainly as a solution, not as the solid, but ice is believed to be a substantial component of the cloud decks of the gas-giant planets Jupiter and Saturn, with sulfur produced by its photolysis responsible for the color of some of those planets' clouds. It can be generated by mixing hydrogen sulfide and ammonia. Preparation Solutions of ammonium hydrosulfide can be prepared by passing hydrogen sulfide gas through concentrated ammonia solution. According to a detailed 1895 report, hydrogen sulfide reacts with concentrated aqueous ammonia solution at room temperature to give . When this species is cooled to 0 °C and treated with additional hydrogen sulfide, one obtains . An ice-cold solution of this substance kept at 0& ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a Jupiter mass, mass more than 2.5 times that of all the other planets in the Solar System combined and slightly less than one-thousandth the mass of the Sun. Its diameter is 11 times that of Earth and a tenth that of the Sun. Jupiter orbits the Sun at a distance of , with an orbital period of . It is the List of brightest natural objects in the sky, third-brightest natural object in the Earth's night sky, after the Moon and Venus, and has been observed since prehistoric times. Its name derives from that of Jupiter (god), Jupiter, the chief deity of ancient Roman religion. Jupiter was the first of the Sun's planets to form, and its inward migration during the primordial phase of the Solar System affected much of the formation history of the other planets. Jupiter's atmosphere consists of 76% hydrogen and 24% helium by mass, with a denser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Compounds
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by Organic compound, organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it. Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted–Lowry acid–base theory, Brønsted acids (proton donors): : The ammonium ion is mildly acidic, reacting with Brønsted bases to return ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Handbook Of Chemistry And Physics
The ''CRC Handbook of Chemistry and Physics'' is a comprehensive one-volume reference resource for science research. First published in 1914, it is currently () in its 105th edition, published in 2024. It is known colloquially among chemists as the "Rubber Bible", as CRC originally stood for "Chemical Rubber Company". As late as the 1962–1963 edition (3604 pages), the ''Handbook'' contained myriad information for every branch of science and engineering. Sections in that edition include: Mathematics, Properties and Physical Constants, Chemical Tables, Properties of Matter, Heat, Hygrometric and Barometric Tables, Sound, Quantities and Units, and Miscellaneous. ''Mathematical Tables from Handbook of Chemistry and Physics'' was originally published as a supplement to the handbook up to the 9th edition (1952); afterwards, the 10th edition (1956) was published separately as '' CRC Standard Mathematical Tables''. Earlier editions included sections such as "Antidotes of Poisons", ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,4-Dinitrochlorobenzene
2,4-Dinitrochlorobenzene (DNCB) is an organic compound with the chemical formula (O2N)2C6H3Cl. It is a yellow solid that is soluble in organic solvents. It is an intermediate for the industrial production of other compounds. Preparation and reactions DNCB is produced commercially by the nitration of ''p''-nitrochlorobenzene with a mixture of nitric and sulfuric acids. Other methods afford the compound less efficiently include the chlorination of 1,3-dinitrobenzene, nitration of o-nitrochlorobenzene and the dinitration of chlorobenzene. By virtue of the two nitro substituents, the chloride in DNCB is particularly susceptible to nucleophilic substitution, at least relative to simple chlorobenzene. In this way, the compound is a precursor to many other compounds. In one example, DNCB is as a substrate in Glutathione S-Transferase, relevant to activity assays. Safety DNCB induces a type IV hypersensitivity reaction in almost all people exposed to it, so it is used medically t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Textile
Textile is an Hyponymy and hypernymy, umbrella term that includes various Fiber, fiber-based materials, including fibers, yarns, Staple (textiles)#Filament fiber, filaments, Thread (yarn), threads, and different types of #Fabric, fabric. At first, the word "textiles" only referred to woven fabrics. However, weaving is not the only manufacturing method, and many other methods were later developed to form textile structures based on their intended use. Knitting and Nonwoven, non-woven are other popular types of fabric manufacturing. In the contemporary world, textiles satisfy the material needs for versatile applications, from simple daily clothing to Bulletproof vest, bulletproof jackets, spacesuits, and Medical gown, doctor's gowns. Textiles are divided into two groups: consumer textiles for domestic purposes and technical textiles. In consumer textiles, Aesthetics (textile), aesthetics and Textile performance#Comfort, comfort are the most important factors, while in techn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals (such as phosphorus) or metalloids (such as arsenic or silicon). These additions produce a range of alloys some of which are harder than copper alone or have other useful properties, such as strength, ductility, or machinability. The archaeological period during which bronze was the hardest metal in widespread use is known as the Bronze Age. The beginning of the Bronze Age in western Eurasia is conventionally dated to the mid-4th millennium BCE (~3500 BCE), and to the early 2nd millennium BCE in China; elsewhere it gradually spread across regions. The Bronze Age was followed by the Iron Age, which started about 1300 BCE and reaching most of Eurasia by about 500 BCE, although bronze continued to be much more widely used than it is in modern times. Because historica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patina
Patina ( or ) is a thin layer that variously forms on the surface of copper, brass, bronze, and similar metals and metal alloys ( tarnish produced by oxidation or other chemical processes), or certain stones and wooden furniture (sheen produced by age, wear, and polishing), or any similar acquired change of a surface through age and exposure. Additionally, the term is used to describe the aging of high-quality leather. The patinas on leather goods are unique to the type of leather, frequency of use, and exposure. Patinas can provide a protective covering to materials that would otherwise be damaged by corrosion or weathering. They may also be aesthetically appealing. Usage On metal, patina is a coating of various chemical compounds such as oxides, carbonates, sulfides, or sulfates formed on the surface during exposure to atmospheric elements (oxygen, rain, acid rain, carbon dioxide, sulfur-bearing compounds). Patina also refers to accumulated changes in surface texture and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Developing
Photographic processing or photographic development is the chemical means by which photographic film or photographic paper, paper is treated after photographic exposure to produce a Negative (photography), negative or Positive (photography), positive image. Photographic processing transforms the latent image into a visible image, makes this permanent and renders it insensitive to light.Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. All processes based upon the gelatin silver process are similar, regardless of the film or paper's manufacturer. Exceptional variations include instant films such as those made by Polaroid Corporation, Polaroid and thermally developed films. Kodachrome required Kodak's proprietary K-14 process. Kodachrome film production ceased in 2009, and K-14 processing is no longer available as of December 30, 2010. Ilfochrome materials use the dye destruction process. Deliberately using the wron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Sulfide008
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) molecular ion with the chemical formula or . It is formed by the addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it. Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): : The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : Thus, the treatment of concentrated solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stink Bomb
A stink bomb, sometimes called a stinkpot, is a device designed to create an unpleasant smell. They range in effectiveness from being used as simple pranks to military grade malodorants or riot control chemical agents. History A stink bomb that could be launched with arrows was invented by Leonardo da Vinci. The 1972 U.S. presidential campaign of Edmund Muskie was disrupted at least four times in Florida in 1972 with the use of stink bombs during the Florida presidential primary. Stink bombs were set off at campaign picnics in Miami and Tampa, at the Muskie campaign headquarters in Tampa and at offices in Tampa where the campaign's telephone bank was located. The stink bomb plantings served to disrupt the picnics and campaign operations, and was deemed by the U.S. Select Committee on Presidential Campaign Activities of the U.S. Senate to have "disrupted, confused, and unnecessarily interfered with a campaign for the office of the Presidency". In 2004, it was reported tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |