|
Aluminium Diboride
Aluminium diboride (AlB2) is a chemical compound made from the metal aluminium and the metalloid boron. It is one of two compounds of aluminium and boron, the other being AlB12, which are both commonly referred to as aluminium boride. Structurally the B atoms form graphite-like sheets with Al atoms between them, and this is very similar to the structure of magnesium diboride. Single crystals of AlB2 exhibit metallic conductivity along the axis parallel to the basal hexagonal plane. Aluminium boride is considered a hazardous substance as it reacts with acids and hydrogen gas to produce toxic gases. For example, it reacts with hydrochloric acid to release borane and aluminium chloride Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are col .... The crystal structure of AlB2 is often used a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure. It was originated by William Burton Pearson and is used extensively in Pearson's handbook of crystallographic data for intermetallic phases. The symbol is made up of two letters followed by a number. For example: * Diamond structure, cF8 * Rutile structure, tP6 Construction The two letters in the Pearson symbol specify the Bravais lattice, and more specifically, the lower-case letter specifies the Crystal system, crystal family, while the upper-case letter the Lattice (group), lattice type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell (atoms which satisfy 1 > x,y,z \geq 0 for the atom's position (x,y,z) in the unit cell). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has a great affinity towards oxygen, passivation (chemistry), forming a protective layer of aluminium oxide, oxide on the surface when exposed to air. It visually resembles silver, both in its color and in its great ability to reflect light. It is soft, magnetism, nonmagnetic, and ductility, ductile. It has one stable isotope, 27Al, which is highly abundant, making aluminium the abundance of the chemical elements, 12th-most abundant element in the universe. The radioactive decay, radioactivity of aluminium-26, 26Al leads to it being used in radiometric dating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloid
A metalloid is a chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and Nonmetal (chemistry), nonmetals. The word metalloid comes from the Latin language, Latin ''metallum'' ("metal") and the Greek language, Greek ''oeides'' ("resembling in form or appearance"). There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature. The six commonly recognised metalloids are boron, silicon, germanium, arsenic, antimony and tellurium. Five elements are less frequently so classified: carbon, aluminium, selenium, polonium and astatine. On a standard periodic table, all eleven elements are in a diagonal region of the p-block extending from boron at the upper left to astatine at lower right. Some periodic tables include a dividing line between metals and nonmetals, and the metalloids may be found cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovas and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is found in smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Dodecaboride
Aluminium dodecaboride () is a superhard chemical compound with 17% aluminium content by weight. It is the hardest boride of the aluminium-boron system, which also includes , , and AlB. Properties There are two crystalline forms, α-, and γ-. Both forms are very similar and consist of a framework with three-dimensional networks of and units. The phase β- is now believed to be the ternary boride . Preparation The β-form can be prepared by the reaction of boron(III) oxide with sulfur and aluminium, then adding carbon to the mixture. Uses The extreme hardness of makes it a favorable component of PCBN inserts, which are mainly used in cutting and grinding to replace diamond or corundum. See also * Boron Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ... * Aluminium borid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
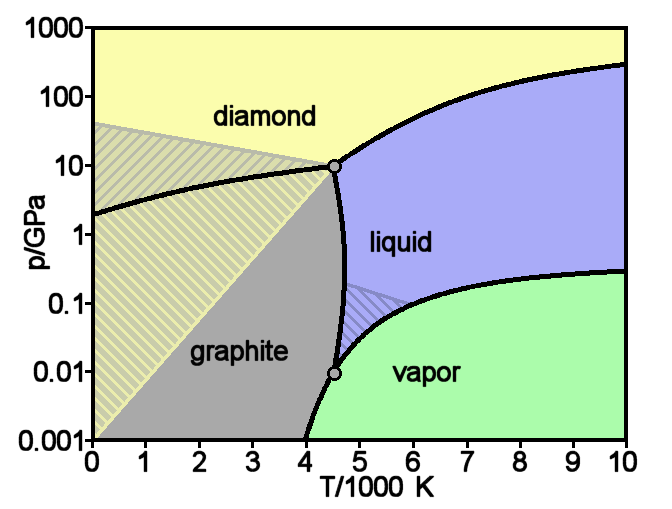
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Diboride
Magnesium diboride is the inorganic compound of magnesium and boron with the formula MgB2. It is a dark gray, water-insoluble solid. The compound becomes superconducting at 39 K (−234 °C), which has attracted attention. In terms of its composition, MgB2 differs strikingly from most low-temperature superconductors, which feature mainly transition metals. Its superconducting mechanism is primarily described by BCS theory. Superconductivity Magnesium diboride's superconducting properties were discovered in 2001. Its critical temperature (''T''c) of is the highest amongst conventional superconductors. Among conventional ( phonon-mediated) superconductors, it is unusual. Its electronic structure is such that there exist two types of electrons at the Fermi level with widely differing behaviours, one of them ( sigma-bonding) being much more strongly superconducting than the other ( pi-bonding). This is at odds with usual theories of phonon-mediated superconductivity which ass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borane
Borane is an inorganic compound with the chemical formula . Because it tends to dimerize or form adducts, borane is very rarely observed. It normally dimerizes to diborane in the absence of other chemicals. It can be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. Structure and properties BH3 is a trigonal planar molecule with D3h symmetry. The experimentally determined B–H bond length is 119 pm. In the absence of other bases, it dimerizes to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction: :BX3 +BH4− → HBX3− + (BH3) (X=F, Cl, Br, I) :2 BH3 → B2H6 The standard enthalpy of dimerization of BH3 is estimated to be −170 kJ mol−1. The boron atom in BH3 has 6 valence electrons. Consequently, it is a strong Lewis acid and reacts with any Lewis base ('L' in equation below) to form an adduct: :BH3 + L � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour. The anhydrous form is commercially important. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. Structure Anhydrous adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |