|
Adiabatic Wall
In thermodynamics, an adiabatic wall between two thermodynamic systems does not allow heat or chemical substances to pass across it, in other words there is no heat transfer or mass transfer. In theoretical investigations, it is sometimes assumed that one of the two systems is the surroundings of the other. Then it is assumed that the work transferred is reversible within the surroundings, but in thermodynamics it is not assumed that the work transferred is reversible within the system. The assumption of reversibility in the surroundings has the consequence that the quantity of work transferred is well defined by macroscopic variables in the surroundings. Accordingly, the surroundings are sometimes said to have a reversible work reservoir. Along with the idea of an adiabatic wall is that of an adiabatic enclosure. It is easily possible that a system has some boundary walls that are adiabatic and others that are not. When some are not adiabatic, then the system is not adiabatically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantity, physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the thermodynamic efficiency, efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), with the third being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
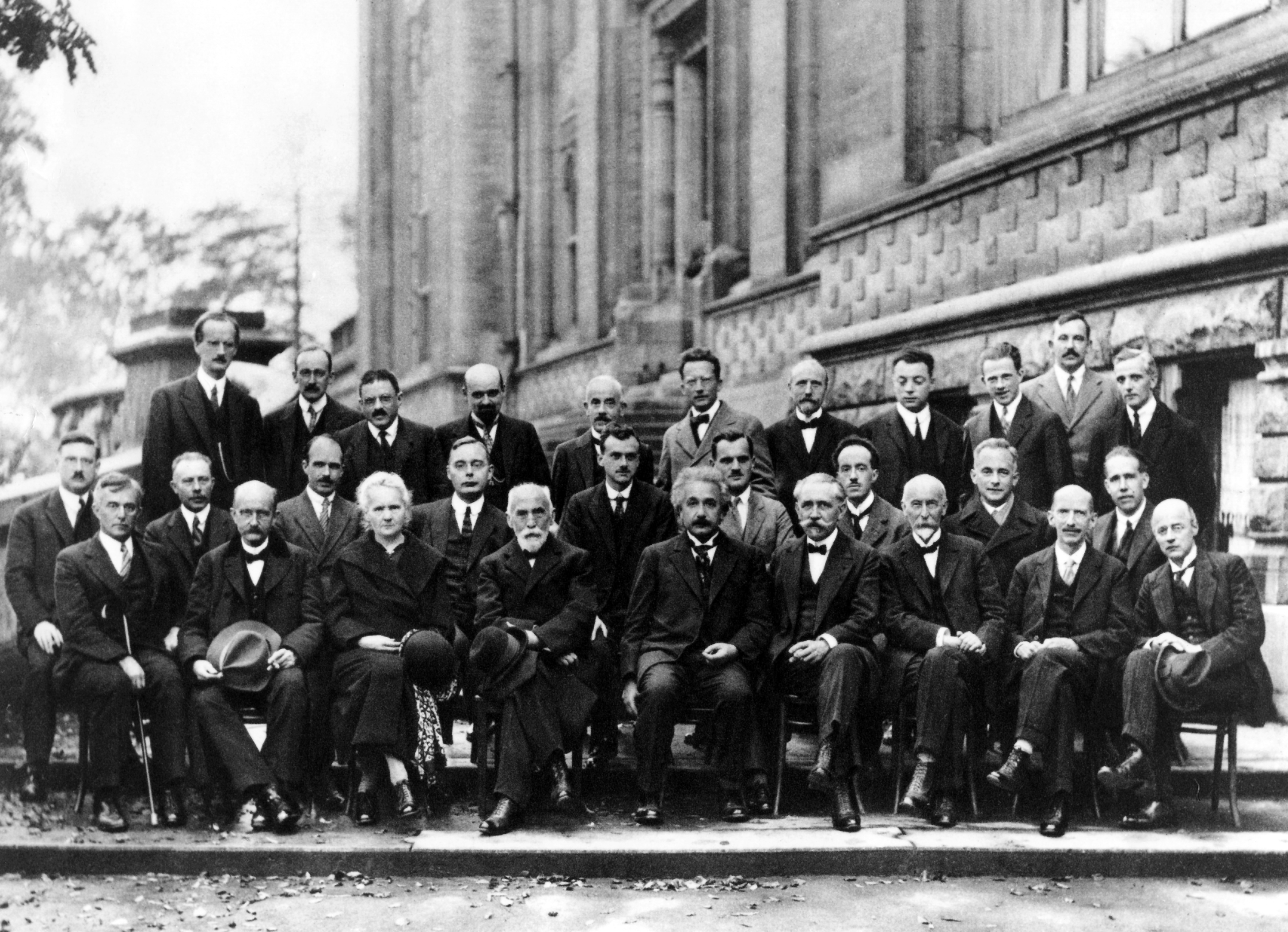
John Gamble Kirkwood
John "Jack" Gamble Kirkwood (May 30, 1907, Gotebo, Oklahoma – August 9, 1959, New Haven, Connecticut) was a noted chemist and physicist, holding faculty positions at Cornell University, the University of Chicago, California Institute of Technology, and Yale University. Early life and background Kirkwood was born in Gotebo, Oklahoma, the oldest child of John Millard and Lillian Gamble Kirkwood. His father was educated as an attorney and was a distributor for the Goodyear Corporation in the state of Kansas. In addition to Jack Kirkwood, there were two younger sisters: Caroline (1910) and Margaret (1921). In 1909, the family moved to Wichita, Kansas. In the 1920s the family traveled to Pasadena, California to escape Midwestern winters. Education While in Pasadena, Kirkwood, age 15, audited chemistry classes at Caltech. Showing remarkable talent in mathematics and chemistry, Kirkwood was persuaded by A. A. Noyes to enroll at Caltech before finishing his high school educati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elsevier
Elsevier ( ) is a Dutch academic publishing company specializing in scientific, technical, and medical content. Its products include journals such as ''The Lancet'', ''Cell (journal), Cell'', the ScienceDirect collection of electronic journals, ''Trends (journals), Trends'', the ''Current Opinion (Elsevier), Current Opinion'' series, the online citation database Scopus, the SciVal tool for measuring research performance, the ClinicalKey search engine for clinicians, and the ClinicalPath evidence-based cancer care service. Elsevier's products and services include digital tools for Data management platform, data management, instruction, research analytics, and assessment. Elsevier is part of the RELX Group, known until 2015 as Reed Elsevier, a publicly traded company. According to RELX reports, in 2022 Elsevier published more than 600,000 articles annually in over 2,800 journals. As of 2018, its archives contained over 17 million documents and 40,000 Ebook, e-books, with over one b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
George H
George may refer to: Names * George (given name) * George (surname) People * George (singer), American-Canadian singer George Nozuka, known by the mononym George * George Papagheorghe, also known as Jorge / GEØRGE * George, stage name of Giorgio Moroder * George, son of Andrew I of Hungary Places South Africa * George, South Africa, a city ** George Airport United States * George, Iowa, a city * George, Missouri, a ghost town * George, Washington, a city * George County, Mississippi * George Air Force Base, a former U.S. Air Force base located in California Computing * George (algebraic compiler) also known as 'Laning and Zierler system', an algebraic compiler by Laning and Zierler in 1952 * GEORGE (computer), early computer built by Argonne National Laboratory in 1957 * GEORGE (operating system), a range of operating systems (George 1–4) for the ICT 1900 range of computers in the 1960s * GEORGE (programming language), an autocode system invented by Charles L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German-British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a number of notable physicists in the 1920s and 1930s. Born shared the 1954 Nobel Prize in Physics with Walther Bothe "for his fundamental research in quantum mechanics, especially in the statistical interpretation of the wave function". Born entered the University of Göttingen in 1904, where he met the three renowned mathematicians Felix Klein, David Hilbert, and Hermann Minkowski. He wrote his PhD thesis on the subject of the stability of elastic wires and tapes, winning the university's Philosophy Faculty Prize. In 1905, he began researching special relativity with Minkowski, and subsequently wrote his habilitation thesis on the Thomson model of the atom. A chance meeting with Fritz Haber in Berlin in 1918 led to discussion of how an io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dewar Flask
A vacuum flask (also known as a Dewar flask, Dewar bottle or thermos) is an insulating storage vessel that slows the speed at which its contents change in temperature. It greatly lengthens the time over which its contents remain hotter or cooler than the flask's surroundings by trying to be as adiabatic as possible. Invented by James Dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. The gap between the two flasks is partially evacuated of air, creating a near-vacuum which significantly reduces heat transfer by conduction or convection. When used to hold cold liquids, this also virtually eliminates condensation on the outside of the flask. Vacuum flasks are used domestically to keep contents inside hot or cold for extended periods of time. They are also used for thermal cooking. Vacuum flasks are also used for many purposes in industry. History The vacuum flask was designed and invented by Scottish scientist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radiation. The first laser was built in 1960 by Theodore Maiman at Hughes Research Laboratories, based on theoretical work by Charles H. Townes and Arthur Leonard Schawlow and the optical amplifier patented by Gordon Gould. A laser differs from other sources of light in that it emits light that is coherence (physics), ''coherent''. Spatial coherence allows a laser to be focused to a tight spot, enabling uses such as optical communication, laser cutting, and Photolithography#Light sources, lithography. It also allows a laser beam to stay narrow over great distances (collimated light, collimation), used in laser pointers, lidar, and free-space optical communication. Lasers can also have high temporal coherence, which permits them to emit light ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calorimetry
In chemistry and thermodynamics, calorimetry () is the science or act of measuring changes in '' state variables'' of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry. Indirect calorimetry calculates heat that living organisms produce by measuring either their production of carbon dioxide and nitrogen waste (frequently ammonia in aquatic organisms, or urea in terrestrial ones), or from their consumption of oxygen. Lavoisier noted in 1780 that heat production can be predicted from oxygen consumption this way, using multiple regression. The dynamic energy budget theory explains why this procedure is corre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Radiation
Thermal radiation is electromagnetic radiation emitted by the thermal motion of particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation. The emission of energy arises from a combination of electronic, molecular, and lattice oscillations in a material. Kinetic energy is converted to electromagnetism due to charge-acceleration or dipole oscillation. At room temperature, most of the emission is in the infrared (IR) spectrum, though above around 525 °C (977 °F) enough of it becomes visible for the matter to visibly glow. This visible glow is called incandescence. Thermal radiation is one of the fundamental mechanisms of heat transfer, along with conduction and convection. The primary method by which the Sun transfers heat to the Earth is thermal radiation. This energy is partially absorbed and scattered in the atmosphere, the latter process being the reason why the sky is visibly blue. Much of the Sun's radiation tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic System
A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics. Thermodynamic systems can be passive and active according to internal processes. According to internal processes, passive systems and active systems are distinguished: passive, in which there is a redistribution of available energy, active, in which one type of energy is converted into another. Depending on its interaction with the environment, a thermodynamic system may be an isolated system, a Closed system#In thermodynamics, closed system, or an Open system (systems theory), open system. An isolated system does not exchange matter or energy with its surroundings. A closed system may exchange heat, experience forces, and exert forces, but does not exchange matter. An open system can interact with its surroundings by exchanging both matter and energy. The physical condition of a thermodynamic system at a given time is described by its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Conduction
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributes this kinetic energy until an object has the same kinetic energy throughout. Thermal conductivity, frequently represented by , is a property that relates the rate of heat loss per unit area of a material to its rate of change of temperature. Essentially, it is a value that accounts for any property of the material that could change the way it conducts heat. Heat spontaneously flows along a temperature gradient (i.e. from a hotter body to a colder body). For example, heat is conducted from the hotplate of an electric stove to the bottom of a saucepan in contact with it. In the absence of an opposing external driving energy source, within a body or between bodies, temperature differences decay over time, and thermal equilibrium is approached, temperature becomin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |