|
ATCvet Code QP53
QP53A Ectoparasiticides for topical use, including insecticides QP53AA Sulfur-containing products :QP53AA01 Mesulfen :QP53AA02 Cymiazol QP53AB Chlorine-containing products :QP53AB01 Clofenotane :QP53AB02 Lindane :QP53AB03 Bromociclen :QP53AB04 Tosylchloramide :QP53AB51 Clofenotane, combinations :QP53AB52 Lindane, combinations QP53AC Pyrethrins and pyrethroids :QP53AC01 Pyrethrum :QP53AC02 Bioallethrin :QP53AC03 Phenothrin :QP53AC04 Permethrin :QP53AC05 Flumethrin :QP53AC06 Cyhalothrin :QP53AC07 Flucythrinate :QP53AC08 Cypermethrin :QP53AC10 Fluvalinate :QP53AC11 Deltamethrin :QP53AC12 Cyfluthrin :QP53AC13 Tetramethrin :QP53AC14 Fenvalerate :QP53AC15 Acrinathrin :QP53AC30 Combinations of pyrethrines :QP53AC51 Pyrethrum, combinations :QP53AC54 Permethrin, combinations :QP53AC55 Flumethrin, combinations QP53AD Amidines :QP53AD01 Amitraz :QP53AD51 Amitraz, combinations QP53AE Carbamates :QP53AE01 Carbaril :QP53AE02 Propoxur :QP53AE03 Bendiocarb QP53AF Organophosphorous compoun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the producti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cypermethrin
Cypermethrin (CP) is a synthetic pyrethroid used as an insecticide in large-scale commercial agricultural applications as well as in consumer products for domestic purposes. It behaves as a fast-acting neurotoxin in insects. It is easily degraded on soil and plants but can be effective for weeks when applied to indoor inert surfaces. Exposure to sunlight, water and oxygen will accelerate its decomposition. Cypermethrin is highly toxic to fish, bees and aquatic insects, according to the National Pesticides Telecommunications Network (NPTN). It is found in many household ant and cockroach killers, including Raid, Ortho, Combat, ant chalk, and some products of Baygon in Southeast Asia. Uses Cypermethrin is used in agriculture to control ectoparasites which infest cattle, sheep, and poultry. Human exposure Cypermethrin is moderately toxic through skin contact or ingestion. It may cause irritation to the skin and eyes. Symptoms of dermal exposure include numbness, tingling, it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
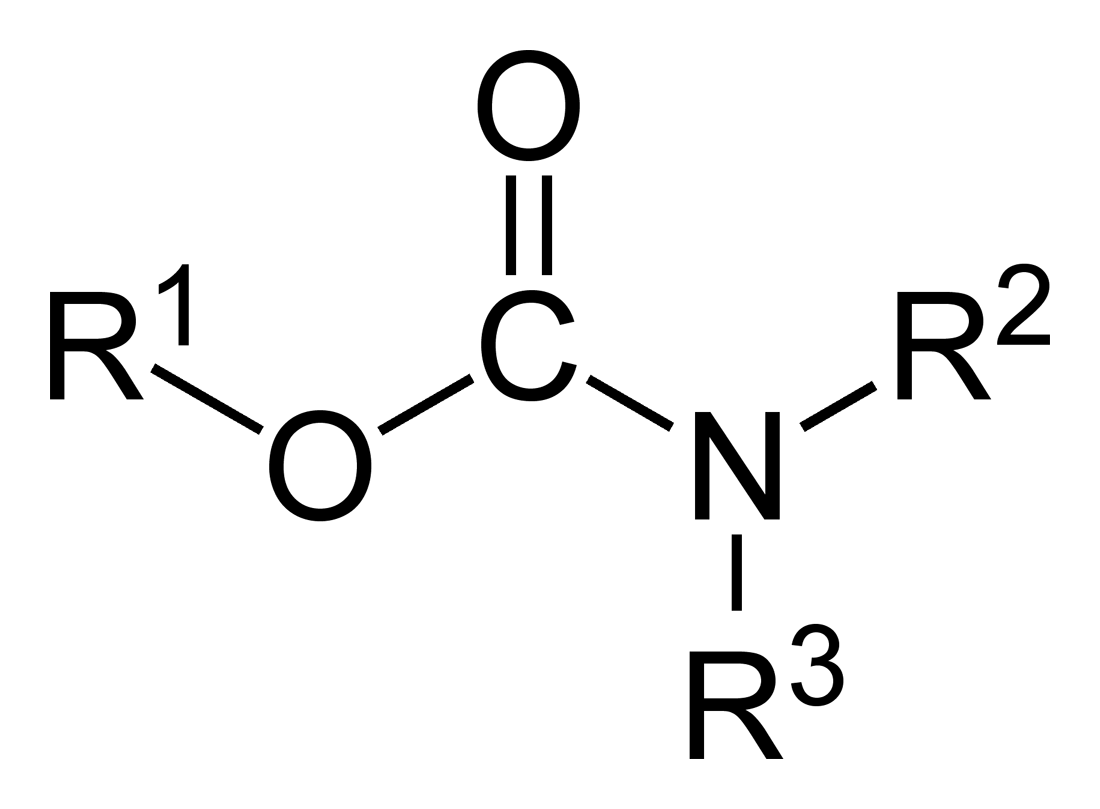
Organophosphorous
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bendiocarb
Bendiocarb is an acutely toxic carbamate insecticide used in public health and agriculture and is effective against a wide range of nuisance and disease vector insects. Many bendiocarb products are or were sold under the tradenames "Ficam" and "Turcam." All bendiocarb-containing products in the United States were recently cancelled, after its manufacturers voluntarily chose to pull their products off the market, rather than conduct additional safety studies required by the EPA.R.E.D. Facts: Bendiocarb U.S. EPA, September 1999. In other countries, it is still used in homes, industrial plants, and food storage sites to control s, |
Propoxur
Propoxur (Baygon) is a carbamate non-systemic insecticide introduced in 1959 with a fast knockdown and long residual effect used against turf, forestry, and household pests and fleas. It is also used in pest control for other domestic animals, ''Anopheles'' mosquitoes, ants, gypsy moths, and other agricultural pests.Budavari, 1996a It can also be used as a molluscicide.EXTOXNET Extension Toxicology Network. Pesticide Information Profile. Propoxur. June 1996. It is produced from catechol. Several U.S. states have petitioned the US Environmental Protection Agency, Environmental Protection Agency (EPA) to use propoxur against Bed bug (insect), bedbug infestations, but the EPA has been reluctant to approve indoor use because of its potential toxicity to children after chronic e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbaril
Carbaryl (1-naphthyl methylcarbamate) is a chemical in the carbamate family used chiefly as an insecticide. It is a white crystalline solid previously sold under the brand name Sevin, which was a trademark of the Bayer Company. The Sevin trademark has since been acquired by GardenTech, which has eliminated carbaryl from most Sevin formulations. Union Carbide discovered carbaryl and introduced it commercially in 1958. Bayer purchased Aventis CropScience in 2002, a company that included Union Carbide pesticide operations. Carbaryl was the third-most-used insecticide in the United States for home gardens, commercial agriculture, and forestry and rangeland protection. As a veterinary drug, it is known as carbaril (INN). Production Carbaryl is often inexpensively produced by direct reaction of methyl isocyanate with 1-naphthol. :C10H7OH + CH3NCO → C10H7OC(O)NHCH3 Alternatively, 1-naphthol can be treated with excess phosgene to produce 1-naphthylchloroformate, which is then con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amitraz
Amitraz (development code BTS27419) is a non-systemic acaricide and insecticideCorta, E., Bakkali, A., Berrueta, L. A., Gallo, B., & Vicente, F. (1999). Kinetics and mechanism of amitraz hydrolysis in aqueous media by HPLC and GC-MS. Talanta, 48(1), 189-199 and has also been described as a scabicide. It was first synthesized by the Boots Co. in England in 1969.Harrison, I. R., et al. (1973). 1,3,5-Triazapenta-1, 4-dienes: Chemical aspects of a new group of pesticides. Pestic. Sci. 4: 901 Amitraz has been found to have an insect repellent effect, works as an insecticide and also as a pesticide synergist.PubChem Substance. Amitraz – Substance Summary. retrieved from https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=24868774#x332 Its effectiveness is traced back on alpha-adrenergic agonist activity, interaction with octopamine receptors of the central nervous system and inhibition of monoamine oxidases and prostaglandin synthesis. Therefore, it leads to overexcitation and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2. Examples of amidines include: * DBU * diminazene * benzamidine * Pentamidine * Paranyline Preparation A common route to primary amidines is the Pinner reaction. Reaction of the nitrile with alcohol in the presence of acid gives an iminoether. Treatment of the resulting compound with ammonia then completes the conversion to the amidine. Instead of using a Bronsted acid, Lewis acids such as aluminium trichloride promote the direct amination of nitriles. They are also generated by amination of an imidoyl chloride. They are also prepared by the addition of organolithium reagents to diimines, followed by protonation or alkylation. Dimethylformamide acetal reacts with primary amines to give amidines: :Me2NC(H)(OMe)2 + RNH2 → Me2NC=NHR + 2 MeOH Propert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrinathrin
Acrinathrin (Rufast and other trade names) is a pyrethroid insecticide and acaricide derived from hexafluoro-2-propanol. In beekeeping, it is used to control the mite ''Varroa jacobsoni ''Varroa jacobsoni'' is a species of mite that parasitises ''Apis cerana'' (Asian honey bees). The more damaging ''Varroa destructor'' was previously included under the name ''V. jacobsoni'', but the two species can be separated on the bas ...'', though resistance is developing. References (cyano-(3-phenoxyphenyl)methyl) 2,2,3-trimethylcyclopropane-1-carboxylates Acaricides Trifluoromethyl compounds {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fenvalerate
Fenvalerate is a synthetic pyrethroid insecticide. It is a mixture of four optical isomers which have different insecticidal activities. The 2-S ''alpha'' (or SS) configuration, known as esfenvalerate, is the most insecticidally active isomer. Fenvalerate consists of about 23% of this isomer. Fenvalerate is an insecticide of moderate mammalian toxicity. In laboratory animals, central nervous system toxicity is observed following acute or short-term exposure. Fenvalerate has applications against a wide range of pests including some of the more destructive such as the ''Helicoverpa assulta''. Residue levels are minimized by low application rates. Fenvalerate is most toxic to bees and fish. It is found in some emulsifiable concentrates, ULV, wettable powders, slow release formulations, insecticidal fogs, and granules. It is most commonly used to control insects in food, feed, and cotton products, and for the control of flies and ticks in barns and stables. Fenvalerate does not affec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethrin
Tetramethrin is a potent synthetic insecticide in the pyrethroid family. It is a white crystalline solid with a melting point of 65-80 °C. The commercial product is a mixture of stereoisomer In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...s. It is commonly used as an insecticide, and affects the insect's nervous system. It is found in many household insecticide products. Tetramethrin has an expected half-life of 12.5-14 days in soil and 13-25 days in water. References External linksPyrethrins and Pyrethroids Fact Sheet - National Pesticide Information Center * Maleimides [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)