|
6-Fluoro-AMT
6-Fluoro-α-methyltryptamine (6-fluoro-AMT, 6F-AMT) is a tryptamine derivative related to compounds such as α-methyltryptamine (AMT) and 5-MeO-AMT which has been sold as a designer drug. Animal tests showed the drug to be somewhat less potent in terms of pharmacological activity than AMT or 5-fluoro-AMT. It produces the head-twitch response, a behavioral proxy of psychedelic-like effects, in rodents. Its for monoamine oxidase A (MAO-A) inhibition is 580 to 1,800nM, compared to 180 to 450nM for 5-fluoro-AMT and 380nM for AMT. 6-Fluoro-AMT was allegedly manufactured and sold from the laboratory operated by Leonard Pickard and Gordon Todd Skinner, who described 6-fluoro-AMT as "a beast". In interviews, Skinner stated that he first began to experiment with 6-fluoro-AMT in the early 1980s by giving it to high school friends. Their experiences made him cautious about the appropriate doses, which he said ranged from 25 to 75mg (Skinner weighed about 250lbs at the time of his own ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
7-Chloro-AMT
7-Chloro-α-methyltryptamine (7-Cl-AMT) is a tryptamine derivative with stimulant effects, invented in the 1960s. It is a weak monoamine oxidase inhibitor but its pharmacology has not otherwise been studied by modern techniques, though several closely related compounds are known to act as serotonin–dopamine releasing agents and agonists of the 5-HT2A receptor. See also * 7-Chlorotryptamine * 5-Chloro-AMT * 5-Chloro-DMT * 5-Fluoro-AMT * 5-Fluoro-AET * 5-Fluoro-DMT * 6-Fluoro-AMT * 7-Methyl-DMT * 7-Methyl-AET * 7F-5-MeO-MET * O-4310 O-4310, also known as 1-isopropyl-6-fluoropsilocin, is a serotonin receptor agonist of the tryptamine family. It is the 1-isopropylated and 6- flourinated derivative of the serotonergic psychedelic psilocin. Pharmacology The drug is said to be ... References Alpha-Alkyltryptamines Chloroarenes Designer drugs Serotonin receptor agonists {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6-Fluoro-DMT
6-Fluoro-DMT, also known as 6-fluoro-''N'',''N''-dimethyltryptamine, is a synthetic serotonin receptor modulator of the tryptamine family. Effects 6-Fluoro-DMT has been said to not be active as a hallucinogen in humans. It has been claimed that this is due to it being " metabolically blocked", though this was not further elaborated on. In the 1960s, it had been theorized by Stephen Szára and colleagues that psychedelic tryptamines were prodrugs that required 6-hydroxylation to become hallucinogenic, but this theory was later found to be incorrect. 6-Fluoro-DMT has been thought to be inactive as a psychedelic in part because 6-fluoro-DET is inactive in terms of such effects. Pharmacology 6-Fluoro-DMT is known to possess varying affinities for serotonin receptors, adrenergic receptors, dopamine receptors, histamine receptors, the imidazoline I1 receptor, sigma receptors, and the serotonin transporter (SERT). It has been found to be a potent partial agonist of the seroto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Fluoro-AET
5-Fluoro-AET, also known as 5-fluoro-α-ethyltryptamine or by the code name PAL-545, is a substituted tryptamine derivative which acts as a serotonin–dopamine releasing agent (SDRA) and as an agonist of the serotonin 5-HT2A receptor. Its values for monoamine release are 36.6nM for serotonin, 5,334nM for norepinephrine, and 150nM for dopamine in rat brain synaptosomes. Its at the serotonin 5-HT2A receptor is 246nM and its at the receptor is 87%. Several close analogues of 5-fluoro-αET, including 5-fluoro-αMT and 5-chloro-αMT, are known to be potent monoamine oxidase inhibitors (MAOIs), specifically of monoamine oxidase A (MAO-A). However, α-ethyltryptamine (αET) is a very weak MAOI. 5-Fluoro-αET has also more recently been assessed, and in contrast to αET, but similarly to drugs like 5-fluoro-αET, was found to be a potent MAOI, with an of 2,480nM. Potent monoamine oxidase inhibition by monoamine releasing agents (MRAs) has been associated with dangerous and some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Tryptamine
Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ALD-52
ALD-52, also known as 1-acetyl-LSD (1A-LSD), is a psychedelic drug of the lysergamide family related to lysergic acid diethylamide (LSD). It has been reported to produce similar psychoactive effects as LSD, but its pharmacological effects on humans are poorly understood. The drug is assumed to act as a prodrug to LSD in humans, and this has been substantiated ''in vitro''. ALD-52 was initially synthesized in 1957 by Albert Hofmann. The purported first confirmed detection of the substance on the illicit market occurred in April 2016. Use and effects In Entry 26 of his compendium '' TiHKAL'', which discussed LSD, chemist Alexander Shulgin touched briefly on the subject of ALD-52. His comments there are vague, second-hand accounts saying doses in the 50–175 μg range have resulted in various conclusions. One account found that there was less visual distortion than with LSD and it seemed to produce less anxiety and tenseness and that it was somewhat less potent. One subj ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
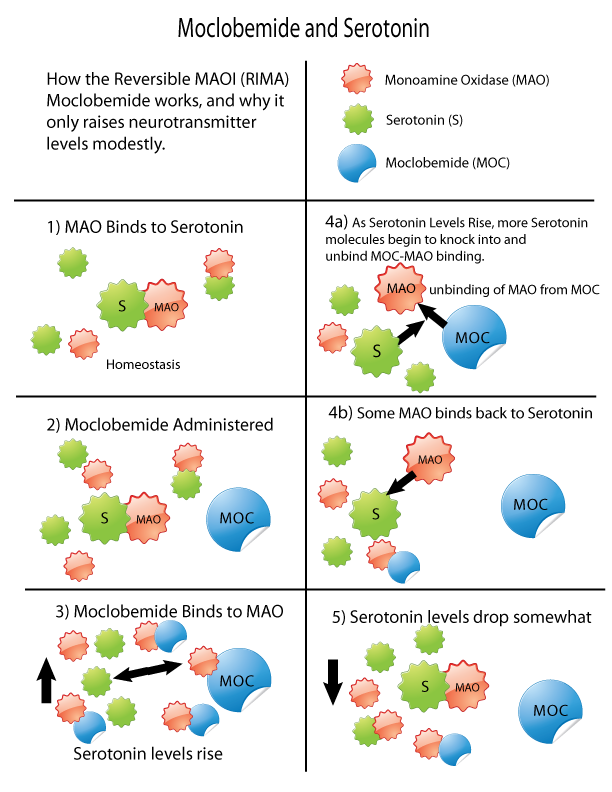
Monoamine Oxidase Inhibitors
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that selectively and reversibly inhibit the MAO-A enzyme. RIMAs are used clinically in the treatment of depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in the United States. Medical uses MAOIs have been found to be effective in the treatment of panic disorder with agoraphob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroarenes
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, bromine Br−1, or iodine I−). Aryl halides are distinct from haloalkanes (alkyl halides) due to significant differences in their methods of preparation, chemical reactivity, and physical properties. The most common and important members of this class are aryl chlorides, but the group encompasses a wide range of derivatives with diverse applications in organic synthesis, pharmaceuticals, and materials science. Classification according to halide Aryl fluorides Aryl fluorides are used as synthetic intermediates, e.g. for the preparation of pharmaceuticals, pesticides, and liquid crystals. The conversion of diazonium salts is a well established route to aryl fluorides. Thus, anilines are precursors to aryl fluorides. In the classic Schiemann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-Alkyltryptamines
Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2A Agonists
Serotonin (), also known as 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter with a wide range of functions in both the central nervous system (CNS) and also peripheral tissues. It is involved in mood, cognition, reward, learning, memory, and physiological processes such as vomiting and vasoconstriction. In the CNS, serotonin regulates mood, appetite, and sleep. Most of the body's serotonin—about 90%—is synthesized in the gastrointestinal tract by enterochromaffin cells, where it regulates intestinal movements. It is also produced in smaller amounts in the brainstem's raphe nuclei, the skin's Merkel cells, pulmonary neuroendocrine cells, and taste receptor cells of the tongue. Once secreted, serotonin is taken up by platelets in the blood, which release it during clotting to promote vasoconstriction and platelet aggregation. Around 8% of the body's serotonin is stored in platelets, and 1–2% is found in the CNS. Serotonin acts as both a vasoconstrictor and v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-4310
O-4310, also known as 1-isopropyl-6-fluoropsilocin, is a serotonin receptor agonist of the tryptamine family. It is the 1-isopropylated and 6- flourinated derivative of the serotonergic psychedelic psilocin. Pharmacology The drug is said to be a serotonin 5-HT2A receptor agonist and to be highly selective for activation of this receptor over the closely related serotonin 5-HT2B and 5-HT2C receptors. Its at the serotonin 5-HT2A receptor was reported to be 5nM and it was said to have an of 89% relative to serotonin. Conversely, O-4310 was said to be inactive at the serotonin 5-HT2B receptor, with no or reported for this receptor, and was claimed to have an of 592nM at the serotonin 5-HT2C receptor with an of approximately 50%. Hence, O-4310 appears to show about 118-fold selectivity for activation of the serotonin 5-HT2A receptor over the serotonin 5-HT2C receptor. The pharmacodynamic activity of O-4310 was briefly described in a patent but not in the published scientifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6-Fluoro-DET
6-Fluoro-DET (6F-DET, 6-fluoro-''N,N''-diethyltryptamine) is a substituted tryptamine derivative related to drugs such as DET and 5-fluoro-DET. It acts as a partial agonist at the 5-HT2A receptor, but while it produces similar physiological effects to psychedelic drugs, it does not appear to produce psychedelic effects itself even at high doses. Relatedly, 6-F-DET does not substituted for LSD in drug discrimination tests and does not produce the head-twitch response in rodents. For the preceding reasons, it saw some use as an active placebo in early clinical trials of psychedelic drugs but was regarded as having little use otherwise, though more recent research into compounds such as AL-34662, TBG and zalsupindole has shown that these kind of non-psychedelic 5-HT2A agonists can have various useful applications. A hypothesis for the lack of head-twitch response in mice and hallucinogenic effects in humans is that 6-F-DET acts as a 5-HT2A receptor partial agonist of the Gq sign ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |