|
2-Hydroxy-1-naphthoic Acid
2-Hydroxy-1-naphthoic acid is an organic compound with the formula C10H6(OH)(CO2H). It is prepared by carboxylation of 2-naphthol by the Kolbe–Schmitt reaction The Kolbe–Schmitt reaction or Kolbe process (named after Hermann Kolbe and Rudolf Schmitt) is a carboxylation chemical reaction that proceeds by treating phenol with sodium hydroxide to form sodium phenoxide, then heating sodium phenoxide wi .... It is one of several hydroxynaphthoic acids. References {{DEFAULTSORT:Hydroxy-1-naphthoic acid, 2- 2-Naphthols Naphthoic acids Alpha hydroxy acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylation
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates. Organic chemistry Carboxylation is a standard conversion in organic chemistry. Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids. Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) – a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid. : Many detailed procedures are d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Naphthol
2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds. Production Traditionally, 2-naphthol is produced by a two-step process that begins with the sulfonation of naphthalene in sulfuric acid:full-text PDF/ref> :C10H8 + H2SO4 → C10H7SO3H + H2O The sulfonic acid group is then cleaved in molten sodium hydroxide: :C10H7(SO3H) + 3 NaOH → C10H7ONa + Na2SO3 + 2 H2O Neutralization of the product with acid gives 2-naphthol. 2-Naphthol can also be produced by a method analogous to the cumene process. 2-Naphthol-derived dyes The Sudan dyes are popular dyes noted for being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
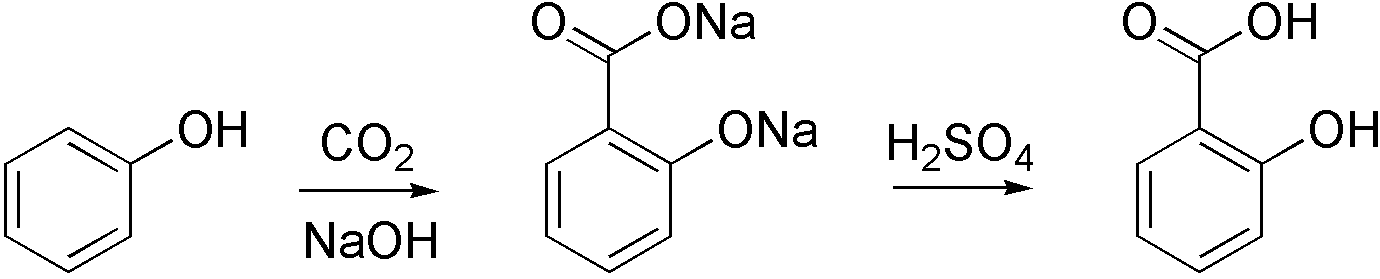
Kolbe–Schmitt Reaction
The Kolbe–Schmitt reaction or Kolbe process (named after Hermann Kolbe and Rudolf Schmitt) is a carboxylation chemical reaction that proceeds by treating phenol with sodium hydroxide to form sodium phenoxide, then heating sodium phenoxide with carbon dioxide under pressure (100 atm, 125 °C), then treating the product with sulfuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid (the precursor to aspirin). 500px, center, The Kolbe–Schmitt reaction By using potassium hydroxide, 4-hydroxybenzoic acid is accessible, an important precursor for the versatile paraben class of biocides used e.g. in personal care products. The methodology is also used in the industrial synthesis of 3-hydroxy-2-naphthoic acid; the regiochemistry of the carboxylation in this case is sensitive to temperature.. Reaction mechanism The Kolbe–Schmitt reaction proceeds via the nucleophilic addition of a phenoxide, classically sodium phenoxide (NaOC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |