Zirconocene dichloride on:
[Wikipedia]
[Google]
[Amazon]
Zirconocene dichloride is an

 The use of trimethylaluminum for this reaction results in exclusive formation of the ''syn''-addition product and, for terminal alkynes, the anti-Markovnikov addition with high selectivity (generally > 10:1). Unfortunately, the use of higher alkylaluminum reagents results in lowered yield, due to the formation of the hydroalumination product (via β-hydrogen elimination of the alkylzirconium intermediate) as side product, and only moderate regioselectivities. Thus, practical applications of the carboalumination reaction are generally confined to the case of methylalumination. Although this is a major limitation, the synthetic utility of this process remains significant, due to the frequent appearance of methyl-substituted alkenes in natural products.
The use of trimethylaluminum for this reaction results in exclusive formation of the ''syn''-addition product and, for terminal alkynes, the anti-Markovnikov addition with high selectivity (generally > 10:1). Unfortunately, the use of higher alkylaluminum reagents results in lowered yield, due to the formation of the hydroalumination product (via β-hydrogen elimination of the alkylzirconium intermediate) as side product, and only moderate regioselectivities. Thus, practical applications of the carboalumination reaction are generally confined to the case of methylalumination. Although this is a major limitation, the synthetic utility of this process remains significant, due to the frequent appearance of methyl-substituted alkenes in natural products.
organozirconium compound
Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have ...
composed of a zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
central atom, with two cyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
and two chloro
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is ...
ligands. It is a colourless diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
solid that is somewhat stable in air.
Preparation and structure
Zirconocene dichloride may be prepared fromzirconium(IV) chloride
Zirconium(IV) chloride, also known as zirconium tetrachloride, () is an inorganic compound frequently used as a precursor to other compounds of zirconium. This white high-melting solid hydrolyzes rapidly in humid air.
Structure
Unlike molecular T ...
-THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
complex and sodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are p ...
:
:ZrCl4(THF)2 + 2 NaCp → Cp2ZrCl2 + 2 NaCl + 2 THF
The closely related compound Cp2ZrBr2 was first described by Birmingham and Wilkinson.
The compound is a bent metallocene In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several ...
: the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being 128°. The Cl-Zr-Cl angle of 97.1° is wider than in niobocene dichloride
Niobocene dichloride is the organometallic compound with the formula (C5H5)2NbCl2, abbreviated Cp2NbCl2. This paramagnetic brown solid is a starting reagent for the synthesis of other organoniobium compounds. The compound adopts a pseudotetrahedr ...
(85.6°) and molybdocene dichloride (82°). This trend helped to establish the orientation of the HOMO
''Homo'' () is the genus that emerged in the (otherwise extinct) genus '' Australopithecus'' that encompasses the extant species ''Homo sapiens'' ( modern humans), plus several extinct species classified as either ancestral to or closely rela ...
in this class of complex.
Reactions
Schwartz's reagent
Zirconocene dichloride reacts withlithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic ...
to give Cp2ZrHCl Schwartz's reagent:
:(C5H5)2ZrCl2 + 1/4 LiAlH4 → (C5H5)2ZrHCl + 1/4 LiAlCl4
Since lithium aluminium hydride is a strong reductant, some over-reduction occurs to give the dihydrido complex, Cp2ZrH2; treatment of the product mixture with methylene chloride converts it to Schwartz's reagent.
Negishi reagent
Zirconocene dichloride can also be used to prepare the Negishi reagent, Cp2Zr( η2-butene
Butene, also known as butylene, is an alkene with the formula . The word ''butene'' may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for ...
), which can be used as a source of Cp2Zr in oxidative cyclisation reactions. The Negishi reagent is prepared by treating zirconocene dichloride with ''n''-BuLi, leading to replacement of the two chloride ligands with butyl group
In organic chemistry, butyl is a four- carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, ...
s. The dibutyl compound subsequently undergoes beta-hydride elimination to give one η2-butene ligand, with the other butyl ligand promptly lost as butane
Butane () or ''n''-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name but ...
via reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
.
Carboalumination
Zirconocene dichloride catalyzes the carboalumination of alkynes bytrimethylaluminum
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially ...
to give a (alkenyl)dimethylalane, a versatile intermediate for further cross coupling reactions for the synthesis of stereodefined trisubstituted olefins. For example, α-farnesene can be prepared as a single stereoisomer by carboalumination of 1-buten-3-yne with trimethylaluminum, followed by palladium-catalyzed coupling of the resultant vinylaluminum reagent with geranyl chloride.
 The use of trimethylaluminum for this reaction results in exclusive formation of the ''syn''-addition product and, for terminal alkynes, the anti-Markovnikov addition with high selectivity (generally > 10:1). Unfortunately, the use of higher alkylaluminum reagents results in lowered yield, due to the formation of the hydroalumination product (via β-hydrogen elimination of the alkylzirconium intermediate) as side product, and only moderate regioselectivities. Thus, practical applications of the carboalumination reaction are generally confined to the case of methylalumination. Although this is a major limitation, the synthetic utility of this process remains significant, due to the frequent appearance of methyl-substituted alkenes in natural products.
The use of trimethylaluminum for this reaction results in exclusive formation of the ''syn''-addition product and, for terminal alkynes, the anti-Markovnikov addition with high selectivity (generally > 10:1). Unfortunately, the use of higher alkylaluminum reagents results in lowered yield, due to the formation of the hydroalumination product (via β-hydrogen elimination of the alkylzirconium intermediate) as side product, and only moderate regioselectivities. Thus, practical applications of the carboalumination reaction are generally confined to the case of methylalumination. Although this is a major limitation, the synthetic utility of this process remains significant, due to the frequent appearance of methyl-substituted alkenes in natural products.
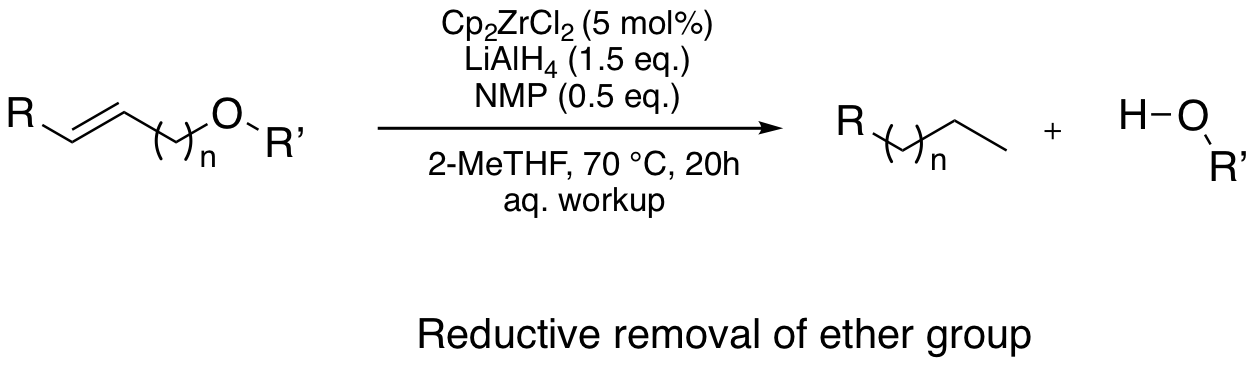
Zr-walk
Zirconocene dichloride together with a reducing reagent can form the zirconocene hydride catalyst in situ, which allows a positional isomerization (so-called "Zr-walk"), and ends up with a cleavage of allylic bonds. Not only individual steps under stoichiometric conditions were described with Schwartz reagent, and Negishi reagent, but also catalytic applications on alkene hydroaluminations, radical cyclisation, polybutadiene cleavage, and reductive removal of functional groups were reported.
References
Further reading
* {{DEFAULTSORT:Zirconocene Dichloride Organozirconium compounds Metallocenes Metal halides Chloro complexes Cyclopentadienyl complexes Zirconium(IV) compounds