Wittig reaction on:
[Wikipedia]
[Google]
[Amazon]
The Wittig reaction or Wittig olefination is a 
 Mechanisms differ for
Mechanisms differ for
 The main limitation of the traditional Wittig reaction is that the reaction proceeds mainly via the
The main limitation of the traditional Wittig reaction is that the reaction proceeds mainly via the 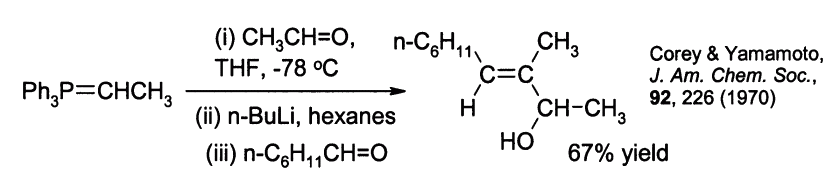

Article
*Wittig reaction in
Article
{{DEFAULTSORT:Wittig Reaction Olefination reactions Carbon-carbon bond forming reactions Name reactions German inventions Homologation reactions 1954 in science 1954 in Germany
chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
of an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
or ketone with a triphenyl phosphonium ylide An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms ...
called a Wittig reagent In organic chemistry, Wittig reagents are organophosphorus compounds of the formula R3P=CHR', where R is usually phenyl. They are used to convert ketones and aldehydes to alkenes:
:
Preparation
Because they typically hydrolyze and oxidize readily ...
. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms chemical bond, bound to a carbon atom, which is connected to the remainder of the molecule by two single bond, single bonds. The group may be re ...
using methylenetriphenylphosphorane
Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species.
Preparation and use
Methylen ...
(Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative.
Stereochemistry
For the reaction with aldehydes, the double bond geometry is readily predicted based on the nature of the ylide. With unstabilised ylides (R3 = alkyl) this results in (''Z'')-alkene product with moderate to high selectivity. With stabilized ylides (R3 = ester or ketone), the (''E'')-alkene is formed with high selectivity. The (''E'')/(''Z'') selectivity is often poor with semistabilized ylides (R3 = aryl). To obtain the (''E'')-alkene for unstabilized ylides, the Schlosser modification of the Wittig reaction can be used. Alternatively, theJulia olefination
The Julia olefination (also known as the Julia–Lythgoe olefination) is the chemical reaction used in organic chemistry of phenyl sulfones (1) with aldehydes (or ketones) to give alkenes (olefins)(3) after alcohol functionalization and reductive ...
and its variants also provide the (''E'')-alkene selectively. Ordinarily, the Horner–Wadsworth–Emmons reaction
The Horner–Wadsworth–Emmons (HWE) reaction is a chemical reaction used in organic chemistry of stabilized phosphonate carbanions with aldehydes (or ketones) to produce predominantly E-alkenes.
In 1958, Leopold Horner published a modifi ...
provides the (''E'')-enoate (α,β-unsaturated ester), just as the Wittig reaction does. To obtain the (''Z'')-enolate, the Still-Gennari modification of the Horner-Wadsworth-Emmons reaction can be used.
Reaction mechanism
Mechanistic studies have focused on unstabilized ylides, because the intermediates can be followed byNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fie ...
. The existence and interconversion of the betaine (3a and 3b) is subject of ongoing research. For lithium-free Wittig reactions, studies support a concerted formation of the oxaphosphetane without intervention of a betaine. In particular, phosphonium ylides 1 react with carbonyl compounds 2 via a +2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
that is sometimes described as having sub>π2s+π2atopology to directly form the oxaphosphetanes 4a and 4b. Under lithium-free conditions, the stereochemistry of the product 5 is due to the kinetically controlled addition of the ylide 1 to the carbonyl 2. When lithium is present, there may be equilibration of the intermediates, possibly via betaine species 3a and 3b. Bruce E. Maryanoff and A. B. Reitz identified the issue about equilibration of Wittig intermediates and termed the process "stereochemical drift". For many years, the stereochemistry of the Wittig reaction, in terms of carbon-carbon bond formation, had been assumed to correspond directly with the Z/E stereochemistry of the alkene products. However, certain reactants do not follow this simple pattern. Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
salts can also exert a profound effect on the stereochemical outcome.
 Mechanisms differ for
Mechanisms differ for aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, ...
and aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s and for aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
and aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, ...
phosphonium ylides. Evidence suggests that the Wittig reaction of unbranched aldehydes under lithium-salt-free conditions do not equilibrate and are therefore under kinetic reaction control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or ...
. E. Vedejs has put forth a theory to explain the stereoselectivity of stabilized and unstabilized Wittig reactions.
Strong evidence indicated that under Li-free conditions, Wittig reactions involving unstabilized (R1= alkyl, H), semistabilized (R1 = aryl), and stabilized (R1 = EWG) Wittig reagents all proceed via a +2retro- +2mechanism under kinetic control, with oxaphosphetane as the one and only intermediate.
Scope and limitations
Functional group tolerance
The Wittig reagents generally toleratecarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
compounds containing several kinds of functional groups such as OH, OR, aromatic nitro
Nitro may refer to:
Chemistry
*Nitrogen, a chemical element and a gas except at very low temperatures, with which many compounds are formed:
**Nitro compound, an organic compound containing one or more nitro functional groups, -NO2
**Nitroalkene, ...
, epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
, and ester groups. Even C=O and nitrile groups can be present if conjugated with the ylide- these are the stabilised ylides mentioned above. Bis-ylides (containing two P=C bonds) have also been made and used successfully. There can be a problem with sterically hindered ketones, where the reaction may be slow and give poor yields, particularly with stabilized ylides, and in such cases the Horner–Wadsworth–Emmons (HWE) reaction (using phosphonate esters) is preferred. Another reported limitation is the often labile nature of aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s, which can oxidize, polymerize or decompose. In a so-called tandem oxidation-Wittig process the aldehyde is formed in situ
''In situ'' (; often not italicized in English) is a Latin phrase that translates literally to "on site" or "in position." It can mean "locally", "on site", "on the premises", or "in place" to describe where an event takes place and is used in ...
by oxidation of the corresponding alcohol.
Stereochemistry
One limitation relates to the stereochemistry of the product. With simple ylides, the product is usually mainly the Z-isomer, although a lesser amount of the E-isomer is often formed also – this is particularly true when ketones are used. If the reaction is performed indimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the maj ...
in the presence of lithium iodide
Lithium iodide, or LiI, is a compound of lithium and iodine. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine. It crystallizes in the NaCl motif. It can participate in various hydrates.Wietelmann, Ulrich ...
or sodium iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions ...
, the product is almost exclusively the Z-isomer. If the E-isomer is the desired product, the Schlosser modification may be used. With stabilised ylides the product is mainly the E-isomer, and this same isomer is also usual with the HWE reaction.
Schlosser modification
 The main limitation of the traditional Wittig reaction is that the reaction proceeds mainly via the
The main limitation of the traditional Wittig reaction is that the reaction proceeds mainly via the erythro
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
betaine
A betaine () in chemistry is any neutral chemical compound with a positively charged cationic functional group, such as a quaternary ammonium or phosphonium cation (generally: onium ions) that bears no hydrogen atom and with a negatively charge ...
intermediate, which leads to the Z-alkene. The erythro betaine can be converted to the threo betaine using phenyllithium
Phenyllithium or lithobenzene is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic synthes ...
at low temperature. This modification affords the E-alkene.
Allylic alcohols can be prepared by reaction of the betaine ylide with a second aldehyde. For example:

Example
An example of its use is in the synthesis of leukotriene A methyl ester. The first step uses a stabilised ylide, where the carbonyl group is conjugated with the ylide preventing self condensation, although unexpectedly this gives mainly the ''cis'' product. The second Wittig reaction uses a non-stabilised Wittig reagent, and as expected this gives mainly the ''cis'' product.
History
The Wittig reaction was reported in 1954 byGeorg Wittig
Georg Wittig (; 16 June 1897 – 26 August 1987) was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Che ...
and his coworker Ulrich Schöllkopf. In part for this contribution, Wittig was awarded the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
in 1979.
See also
* Corey–Chaykovsky reagent *Horner–Wadsworth–Emmons reaction
The Horner–Wadsworth–Emmons (HWE) reaction is a chemical reaction used in organic chemistry of stabilized phosphonate carbanions with aldehydes (or ketones) to produce predominantly E-alkenes.
In 1958, Leopold Horner published a modifi ...
* Julia olefination
The Julia olefination (also known as the Julia–Lythgoe olefination) is the chemical reaction used in organic chemistry of phenyl sulfones (1) with aldehydes (or ketones) to give alkenes (olefins)(3) after alcohol functionalization and reductive ...
* Peterson olefination
* Tebbe's reagent
Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It ...
* Organophosphorus chemistry
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective in ...
* Homologation reaction
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant ...
* Kauffmann olefination
* Titanium–zinc methylenation
References
External links
*Wittig reaction inOrganic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and ex ...
, Coll. Vol. 10, p. 703 (2004); Vol. 75, p. 153 (1998).Article
*Wittig reaction in
Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and ex ...
, Coll. Vol. 5, p. 361 (1973); Vol. 45, p. 33 (1965).Article
{{DEFAULTSORT:Wittig Reaction Olefination reactions Carbon-carbon bond forming reactions Name reactions German inventions Homologation reactions 1954 in science 1954 in Germany