Superbase on:
[Wikipedia]
[Google]
[Amazon]
A superbase is a compound that has a particularly high affinity for
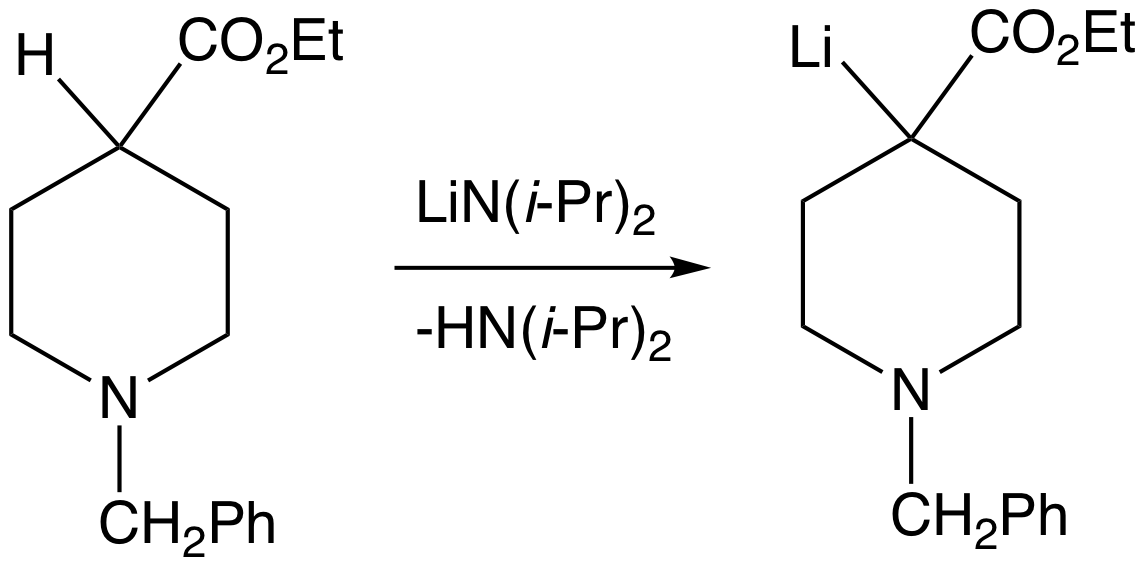 Organometallic compounds of
Organometallic compounds of
protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
. Superbases are of theoretical interest and potentially valuable in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009.
Definitions
GenericallyIUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
defines a superbase as a "compound having a very high basicity
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. ...
, such as lithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature ...
." Superbases are often defined in two broad categories, organic and organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
.
Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge
1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula CH(NMe) (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional ...
(pKBH+ = 18.6 in MeCN)." In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines includ ...
, guanidine
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experie ...
, and phosphazene Phosphazenes refer to classes of organophosphorus compounds featuring phosphorus(V) with a double bond between P and N. One class of phosphazenes have the formula . These phosphazenes are also known as iminophosphoranes and phosphine imides. They a ...
functional groups. Strong superbases can be designed by utilizing multiple intramolecular hydrogen bonds that stabilize the conjugate acid.
Organometallic superbases, sometimes called Lochmann–Schlosser superbases, result from the combination of alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
alkoxides
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, w ...
and organolithium
In organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, ...
reagents. Caubère defines superbases as "bases resulting from a mixing of two (or more) bases leading to new basic species possessing inherent new properties. The term ''superbase'' does not mean a base is thermodynamically and/or kinetically stronger than another, instead it means that a basic reagent is created by combining the characteristics of several different bases."
Organic superbases
290 px, left, Protonation of Verkade base. Its conjugate acid has a pKa of 32.9 in acetonitrile. Organic superbases are mostly charge-neutral, nitrogen containing species, where nitrogen act as a proton acceptor. These include thephosphazene Phosphazenes refer to classes of organophosphorus compounds featuring phosphorus(V) with a double bond between P and N. One class of phosphazenes have the formula . These phosphazenes are also known as iminophosphoranes and phosphine imides. They a ...
s, phosphanes, amidines
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines includ ...
, and guanidines
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experie ...
. Other organic compounds that meet the physicochemical or structural definitions of 'superbase' include proton chelators like the aromatic proton sponge
1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula CH(NMe) (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional ...
s and the bispidines. Multicyclic polyamines
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, Hygroscopy, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium d ...
, like DABCO
DABCO (1,4-diazabicyclo .2.2ctane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent i ...
might also be loosely included in this category.'' Phosphanes and carbodiphosphoranes are also strong organosuperbases. ''
Despite enormous proton affinity, the organosuperbases can exhibit low nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
.
Organometallic
 Organometallic compounds of
Organometallic compounds of electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
metals are superbases, but they are generally strong nucleophiles. Examples include organolithium
In organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, ...
and organomagnesium (Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
) compounds. Another type of organometallic superbase has a reactive metal exchanged for a hydrogen on a heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecula ...
, such as oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
(unstabilized alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
s) or nitrogen (metal amides
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
such as lithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature ...
).
The Schlosser base (or Lochmann-Schlosser base), the combination of ''n''-butyllithium and potassium ''tert''-butoxide, is commonly cited as a superbase. ''n''-Butyllithium and potassium ''tert''-butoxide form a mixed aggregate of greater reactivity than either component reagent.
Inorganic
Inorganic superbases are typicallysalt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
-like compounds with small, highly charged anions, e.g. lithium hydride
Lithium hydride is an inorganic compound with the formula Li H. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not solub ...
, potassium hydride
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although commercial samples appear gray. It is a powerful superbase that is useful in organic synthesis. It is sold com ...
, and sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in co ...
. Such species are insoluble, but the surfaces of these materials are highly reactive and slurries
A slurry is a mixture of denser solids suspended in liquid, usually water. The most common use of slurry is as a means of transporting solids or separating minerals, the liquid being a carrier that is pumped on a device such as a centrifugal pu ...
are useful in synthesis.
Applications
Superbases are used inorganocatalysis
In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic com ...
.{{how, date=November 2022
See also
*Superacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid ...
*Phosphazene Phosphazenes refer to classes of organophosphorus compounds featuring phosphorus(V) with a double bond between P and N. One class of phosphazenes have the formula . These phosphazenes are also known as iminophosphoranes and phosphine imides. They a ...
References