A superalloy, or high-performance alloy, is an
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
with the ability to operate at a high fraction of its melting point.
Several key characteristics of a superalloy are excellent
mechanical strength
The field of strength of materials, also called mechanics of materials, typically refers to various methods of calculating the stresses and strains in structural members, such as beams, columns, and shafts. The methods employed to predict the re ...
, resistance to
thermal creep deformation, good surface stability, and resistance to
corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
or
oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
.
The crystal structure is typically
face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
(FCC)
austenitic
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 ...
. Examples of such alloys are
Hastelloy
Haynes International, Inc., headquartered in Kokomo, Indiana, is one of the largest producers of corrosion-resistant and high-temperature alloys. In addition to Kokomo, Haynes has manufacturing facilities in Arcadia, Louisiana, and Mountain Home ...
,
Inconel
Inconel is a registered trademark of Special Metals Corporation for a family of austenitic nickel-chromium-based superalloys.
Inconel alloys are oxidation-corrosion-resistant materials well suited for service in extreme environments subjected ...
,
Waspaloy
Waspaloy is a registered trademark of United Technologies Corp that refers to an age hardening austenitic (face-centred cubic) nickel-based superalloy. Waspaloy is typically used in high temperature applications, particularly in gas turbines.
...
,
Rene alloys,
Incoloy
Incoloy refers to a range of superalloys now produced by the Special Metals Corporation (SMC) group of companies and created with a trademark by the Inco company in 1952. Originally Inco protected these alloys by patent. In 2000, the SMC published ...
,
MP98T,
TMS alloys, and
CMSX single crystal alloys.
Superalloy development has relied heavily on both chemical and process innovations. Superalloys develop high temperature strength through
solid solution strengthening
In metallurgy, solid solution strengthening is a type of alloying that can be used to improve the strength of a pure metal. The technique works by adding atoms of one element (the alloying element) to the crystalline lattice of another element ...
and
precipitation strengthening from secondary phase precipitates such as gamma prime and carbides. Oxidation or corrosion resistance is provided by elements such as
aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
and
chromium. Superalloys are often cast as a single crystal—while
grain boundaries
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional defects in the crystal structure, and tend to decrease the electrical and thermal ...
may provide strength at low temperatures, they decrease creep resistance.
The primary application for such alloys is in aerospace and marine
turbine engine
A gas turbine, also called a combustion turbine, is a type of continuous flow internal combustion engine. The main parts common to all gas turbine engines form the power-producing part (known as the gas generator or core) and are, in the directi ...
s. Creep is typically the lifetime-limiting factor in gas turbine blades.
Superalloys are the materials which have made much of very-high-temperature engineering technology possible.
Chemical development
Because these alloys are intended for high temperature applications (i.e. holding their shape at temperatures near their melting point) their
creep and oxidation resistance are of primary importance.
Nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
(Ni)-based superalloys have emerged as the material of choice for these applications because of their unique γ' precipitates.
The properties of these Ni-based superalloys can be tailored to a certain extent through the addition of many other elements, both common and exotic, including not only
metals
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typical ...
, but also
metalloids and
nonmetals;
chromium,
iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
,
cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
,
molybdenum,
tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
,
tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that ...
,
aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
,
titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
,
zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
,
niobium,
rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
,
yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a " rare-earth element". Yttrium is almost always found in co ...
,
vanadium,
carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
,
boron or
hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri M ...
are some examples of the alloying additions used. Each of these additions has been chosen to serve a particular purpose in optimizing the properties for high temperature application.
Creep resistance is dependent, in part, on slowing the speed of
dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to s ...
motion within a crystal structure. In modern Ni-based superalloys, the γ’-Ni
3(Al,Ti)
phase
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
* Phase space, a mathematic ...
present acts as a barrier to dislocation motion. For this reason, this γ’
intermetallic
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic eleme ...
phase, when present in high volume fractions, drastically increases the strength of these alloys due to its ordered nature and high coherency with the γ matrix. The chemical additions of
aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
and
titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
promote the creation of the γ’ phase. The γ’ phase size can be precisely controlled by careful precipitation strengthening heat treatments. Many superalloys are produced using a two-phase heat treatment that creates a dispersion of cuboidal γ’ particles known as the primary phase, with a fine dispersion between these known as secondary γ’. In order to improve the oxidation resistance of these alloys, Al, Cr, B, and Y are added. The Al and Cr form oxide layers that passivate the surface and protect the superalloy from further oxidation while B and Y are used to improve the adhesion of this oxide scale to the substrate. Cr, Fe, Co, Mo and Re all preferentially partition to the γ matrix while Al, Ti, Nb, Ta, and V preferentially partition to the γ’ precipitates and solid solution strengthen the matrix and precipitates respectively. In addition to solid solution strengthening, if grain boundaries are present, certain elements are chosen for grain boundary strengthening. B and Zr tend to segregate to the grain boundaries which reduces the grain boundary energy and results in better grain boundary cohesion and ductility. Another form of grain boundary strengthening is achieved through the addition of C and a carbide former, such as Cr, Mo, W, Nb, Ta, Ti, or Hf, which drives precipitation of carbides at grain boundaries and thereby reduces grain boundary sliding.
Active research
While Ni-based superalloys are excellent high temperature materials and have proven very useful, Co-based superalloys potentially possess superior hot corrosion, oxidation, and wear resistance as compared to Ni-based superalloys. For this reason, efforts have also been put into developing Co-based superalloys over the past several years. Despite that, traditional Co-based superalloys have not found widespread usage because they have a lower strength at high temperature than Ni-based superalloys.
The main reason for this is that—until recently—they appeared to lack the γ’ precipitation strengthening that is so important in the high temperature strength of Ni-based superalloys. A 2006 report on metastable γ’-Co
3(Al,W) intermetallic compound with the L1
2 structure suggests Co-based alloys as alternative to traditional Ni-based superalloys. However this class of alloys was reported in a PhD thesis by C. S. Lee in 1971.
The two-phase microstructure consists of cuboidal γ’ precipitates embedded in a continuous γ matrix and is therefore morphologically identical to the microstructure observed in Ni-based superalloys. Like in the Ni-based system, there is a high degree of coherency between the two phases, which is one of the main factors resulting in the superior strength at high temperatures.
This provides a pathway for the development of a new class of load-bearing Co-based superalloys for application in severe environments. In these alloys, W is the crucial addition for forming the γ’ intermetallic compound; this makes them much denser (>9.6 g/cm
3) compared to Ni-based superalloys. Recently a new class of γ - γ’ cobalt-based superalloys have been developed that are W-free and have much lower density comparable to nickel-based superalloys.
In addition to the fact that many of the properties of these new Co-based superalloys could be better than those of the more traditional Ni-based ones, Co also has a higher melting temperature than Ni. Therefore, if the high temperature strength could be improved, the development of novel Co-based superalloys could allow for an increase in jet engine operation temperature resulting in an increased efficiency.
Phase formation
Adding new elements is usually good because of solid solution strengthening, but engineers need to be careful about which phases precipitate. Precipitates can be classified as geometrically close-packed (GCP),
topologically close-packed (TCP), or carbides. GCP phases are usually good for mechanical properties, but TCP phases are often deleterious. Because TCP phases are not truly close packed, they have few slip systems and are very brittle. They are additionally bad because they "scavenge" elements away from GCP phases. Many elements that are good for forming γ' or have great solid solution strengthening may precipitate TCPs. Engineers need to find the balance that promotes GCPs while avoiding TCPs.
An area of the alloy with TCP phase formation will be weak because:
* the TCP phase has inherently poor mechanical properties
* the TCP phase is incoherent with the γ matrix
* the TCP phase is surrounded by a "depletion zone" where there is no γ'
* the TCP phase usually forms sharp plate or needle-like morphologies which easily nucleate cracks
The main GCP phase is γ'. Almost all superalloys are Ni-based because of this phase. γ' is an ordered L1 (pronounced L-one-two), which means it has a certain atom on the face of the unit cell, and a certain atom on the corners of the unit cell. For Ni-based superalloys, that usually means Ni on the faces and Ti or Al on the corners.
Another "good" GCP phase is γ
''. It is also coherent with γ, but it dissolves at high temperatures.
Families of superalloys
History and development of Ni-based superalloys
The United States became interested in gas turbine development around 1905.
From 1910-1915, austenitic ( γ phase) stainless steels were developed for the high temperatures in gas turbines. By 1929, 80Ni-20Cr alloy was the norm, with small additions of Ti and Al. Although early metallurgists did not know it yet, they were forming small γ' precipitates in Ni-based superalloys. These alloys quickly surpassed Fe- and Co-based superalloys, which were strengthened by carbides and solid solution strengthening.
Although Cr was great for protecting the alloys from oxidation and corrosion up to 700 °C, metallurgists began decreasing Cr in favor of Al, which had oxidation resistance at much higher temperatures. The lack of Cr caused issues with hot corrosion, so coatings needed to be developed.
Around 1950,
vacuum melting became commercialized, which allowed metallurgists to create higher purity alloys with more precise composition.
In the 60s and 70s, metallurgists changed focus from alloy chemistry to alloy processing.
Directional solidification
Directional solidification (DS) and progressive solidification are types of solidification within castings. Directional solidification is solidification that occurs from farthest end of the casting and works its way towards the sprue. Progressiv ...
was developed to allow columnar or even single-crystal turbine blades.
Oxide dispersion strengthening could obtain very fine grains and
superplasticity
In materials science, superplasticity is a state in which solid crystalline material is deformed well beyond its usual breaking point, usually over about 600% during tensile deformation. Such a state is usually achieved at high homologous tempe ...
.
Ni-based superalloy phases
* Gamma (γ): This phase composes the matrix of Ni-based superalloy. It is a solid solution fcc austenitic phase of the alloying elements.
Alloying elements found in most commercial Ni-based alloys are, C, Cr, Mo, W, Nb, Fe, Ti, Al, V, and Ta. During the formation of these materials, as the Ni-alloys are cooled from the melt, carbides begin to precipitate, at even lower temperatures γ' phase precipitates.
* Gamma prime (γ'): This phase constitutes the precipitate used to strengthen the alloy. It is an
intermetallic
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic eleme ...
phase based on Ni
3(Ti,Al) which have an ordered FCC L1
2 structure.
The γ' phase is coherent with the matrix of the superalloy having a lattice parameter that varies by around 0.5%. Ni
3(Ti,Al) are ordered systems with Ni atoms at the cube faces and either Al or Ti atoms at the cube edges. As particles of γ' precipitates aggregate, they decrease their energy states by aligning along the <100> directions forming cuboidal structures.
This phase has a window of instability between 600 °C and 850 °C, inside of which γ' will transform into the HCP η phase. For applications at temperatures below 650 °C, the γ" phase can be utilized for strengthening.
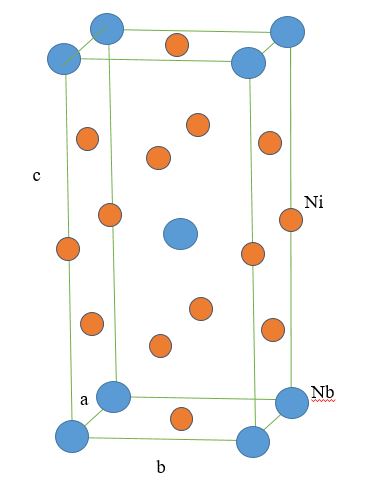
* Gamma double prime (γ"): This phase typically possesses the composition of Ni
3Nb or Ni
3V and is used to strengthen Ni-based superalloys at lower temperatures (<650 °C) relative to γ'. The crystal structure of γ" is
body-centered tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a squa ...
(BCT), and the phase precipitates as 60 nm by 10 nm discs with the (001) planes in γ" parallel to the family in γ. These
anisotropic discs form as a result of
lattice mismatch between the
BCT precipitate and the
FCC
The Federal Communications Commission (FCC) is an independent agency of the United States federal government that regulates communications by radio, television, wire, satellite, and cable across the United States. The FCC maintains jurisdictio ...
matrix. This
lattice mismatch leads to high
coherency strains which, together with
order hardening, comprise the primary strengthening mechanisms. The γ" phase is unstable above approximately 650 °C.
[Dunand, David C. "Materials Science & Engineering 435: High Temperature Materials". Northwestern University, Evanston. 25 February 2016. Lecture.]
* Carbide phases: Carbide formation is usually considered deleterious although in Ni-based superalloys they are used to stabilize the structure of the material against deformation at high temperatures. Carbides form at the grain boundaries inhibiting grain boundary motion.
*Topologically close-packed (TCP) phases: The term
"TCP phase" refers to any member of a family of phases (including the σ phase, the χ phase, the μ phase, and the
Laves phase
Laves phases are intermetallic phases that have composition AB2 and are named for Fritz Laves who first described them. The phases are classified on the basis of geometry alone. While the problem of packing spheres of equal size has been well- ...
) which are not atomically close-packed but possess some close-packed planes with
HCP stacking. TCP phases are characterized by their tendency to be highly brittle and deplete the γ matrix of strengthening,
solid solution
A solid solution, a term popularly used for metals, is a homogenous mixture of two different kinds of atoms in solid state and have a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The wor ...
refractory elements (including Cr, Co, W, and Mo). These phases form as a result of kinetics after long periods of time (thousands of hours) at high temperatures (>750 °C).
History and development of Co-based superalloys
Historically, Co-based superalloys have depended on carbide precipitation and solid solution strengthening for mechanical properties. While these strengthening mechanisms are inferior to gamma prime (γ') precipitation strengthening,
cobalt has a higher melting point than currently ubiquitous nickel-based superalloys and has superior hot corrosion resistance and thermal fatigue. As a result, carbide-strengthened Co-based superalloys are used in lower stress, higher temperature applications such as stationary vanes in gas turbines.
However, recent research has shown that cobalt ''can'' exhibit the γ' phase. Actually, the first reported existence of γ' occurred in a 1971 PhD dissertation,
but was never published. The γ/γ' microstructure was rediscovered and first published in 2006 by Sato et al.
That γ' phase was Co
3(Al, W). It was furthermore found that Mo, Ti, Nb, V, and Ta partition to the γ' phase, while Fe, Mn, and Cr partition to the matrix γ.
The next family of Co-based superalloys was discovered in 2015 by Makineni et al. This family has a similar γ/γ' microstructure, but is tungsten-free and has a γ' phase of Co
3(Al,Mo,Nb).
Since tungsten is a very heavy element, the elimination of tungsten makes Co-based alloys increasingly viable in turbines for aircraft, where low density is especially important.
The most recently discovered family of superalloys was computationally predicted in a high throughput study by Nyshadham et al. in 2017, and demonstrated in the lab by Reyes Tirado et al. in 2018.
This γ' phase is again tungsten free and has the composition Co
3(Nb,V) and Co
3(Ta,V).
Co-based superalloy phases
*Gamma (γ): As in Ni-based superalloys, this is the matrix phase. While Co-based superalloys are not used commercially to the extent of Ni-based superalloys, alloying elements found in research Co-based alloys are C, Cr, W, Ni, Ti, Al, Ir, and Ta.
As in stainless steels, Chromium is used (occasionally up to 20 wt.%) to improve resistance to oxidation and corrosion via the formation of a Cr
2O
3 passive layer, which is critical for use in gas turbines, but also provides solid-solution strengthening due to the mismatch in the atomic radii of Co and Cr, and precipitation hardening due to the formation of MC-type carbides.
* Gamma Prime (γ'): As in Ni-based superalloys, this phase constitutes the precipitate used to strengthen the alloy. It is usually close-packed with a L1
2 structure of Co
3Ti or FCC Co
3Ta, though both W and Al have been found to integrate into these cuboidal precipitates quite well. The elements Ta, Nb, and Ti integrate into the γ’ phase and are quite effective at stabilizing it at high temperatures.
* Carbide Phases: As is common with carbide formation, carbides strengthen the alloy through precipitation hardening but decrease low-temperature ductility.
* Topologically Close-Packed (TCP) phases may also appear in some developmental Co-based superalloys, but embrittle the alloy and are thus undesirable.
Fe-based superalloy phases
The use of steels in superalloy applications is of interest because certain steel alloys have showed creep and oxidation resistance similar to that of Ni-based superalloys, while being far less expensive to produce.
Gamma (γ): Like the phases found in Ni-based superalloys, Fe-based alloys feature a matrix phase of austenite iron (FCC). Alloying elements that are commonly found in these stainless steel alloys include: Al, B, C, Co, Cr, Mo, Ni, Nb, Si, Ti, W, and Y. While Al is introduced for its oxidation benefits, Al additions must be kept at low weight fractions (wt.%) because Al stabilizes a ferritic (BCC) primary phase matrix, which is an undesirable phase in superalloy microstructures, as it is inferior to the high temperature strength exhibited by an austenitic (FCC) primary phase matrix.
Gamma-prime (γ’): This phase is introduced as precipitates to strengthen the alloy. Like in Ni-based alloys, γ’-Ni3Al precipitates can be introduced with the proper balance of Al, Ni, Nb, and Ti additions.
Microstructure of Fe-based superalloys
Two major types of austenitic stainless steels exist and are characterized by the oxide layer that forms at the surface of the steel: chromia-forming or alumina-forming stainless steel. Chromia-forming stainless steel is the most common type of stainless steel produced. However, chromia-forming steels do not exhibit high creep resistance at high operating temperatures, especially in environments with water vapor, when compared to Ni-based superalloys. Exposure to water vapor at high operating temperatures can result in an increase in internal oxidation in chromia-forming alloys and rapid formation of volatile Cr (oxy)hydroxides, both of which can reduce the durability and lifetime of the alloy.
Alumina-forming austenitic stainless steels feature a single-phase matrix of austenite iron (FCC) with an alumina oxide at the surface of the steel. Alumina is more thermodynamically stable in oxygen than chromia. More commonly, however, precipitate phases are introduced to increase strength and creep resistance. In alumina-forming steels, NiAl precipitates are introduced to act as Al reservoirs to maintain the protective alumina layer. In addition, Nb and Cr additions help form and stabilize alumina by increasing precipitate volume fractions of NiAl.
Research endeavors for the development of alumina-forming, Fe-base superalloys have shown at least 5 grades of alumina-forming austenitic (AFA) alloys, with different operating temperatures at oxidation in air + 10% water vapor:
* AFA Grade: (50-60)Fe-(20-25)Ni-(14-15)Cr-(2.5-3.5)Al-(1-3)Nb wt.% base
** 750-800 °C operating temperatures at oxidation in air + 10% water vapor
* Low Nickel AFA Grade: 63Fe-12Ni-14Cr-2.5Al-0.6Nb-5Mn3Cu wt.% base
** 650 °C operating temperatures at oxidation in air + 10% water vapor
* High Performance AFA Grade: (45-55)Fe-(25-30)Ni-(14-15)Cr(3.5-4.5)Al-(1-3)Nb-(0.02-0.1)Hf/Y wt.% base
** 850-900 °C operating temperatures at oxidation in air + 10% water vapor
* Cast AFA Grade: (35-50)Fe-(25-35)Ni-14Cr-(3.5-4)Al-1Nb wt.% base
** 750-1100 °C operating temperatures at oxidation in air + 10% water vapor, depending upon Ni wt.%
* AFA superalloy (40-50)Fe-(30-35)Ni-(14-19)Cr-(2.5-3.5)Al-3Nb
** 750-850 °C operating temperatures at oxidation in air + 10% water vapor
Operating temperatures with oxidation in air and no water vapor are expected to be higher. In addition, an AFA superalloy grade was shown to exhibit a creep strength approaching that of the nickel-based alloy UNS N06617.
Microstructure of superalloys
In pure Ni
3Al phase
atoms of aluminium are placed at the vertices of the cubic cell and form the sublattice A. Atoms of nickel are located at centers of the faces and form the sublattice B. The phase is not strictly
stoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equ ...
. There may exist an excess of vacancies in one of the sublattices, which leads to deviations from stoichiometry. Sublattices A and B of the γ'-phase can solute a considerable proportion of other elements. The alloying elements are dissolved in the γ-phase as well. The γ'-phase hardens the alloy through an unusual mechanism called the
yield strength anomaly.
Dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to s ...
s dissociate in the γ'-phase, leading to the formation of an
anti-phase boundary. At elevated temperature, the free energy associated with the anti-phase boundary (APB) is considerably reduced if it lies on a particular plane, which by coincidence is not a permitted slip plane. One set of partial dislocations bounding the APB cross-slips so that the APB lies on the low-energy plane, and, since this low-energy plane is not a permitted slip plane, the dissociated dislocation is now effectively locked. By this mechanism, the yield strength of γ'-phase Ni
3Al actually ''increases'' with temperature up to about 1000 °C, giving superalloys their currently unrivaled high-temperature strength.
Initial material selection for blade applications in
gas turbine
A gas turbine, also called a combustion turbine, is a type of continuous flow internal combustion engine. The main parts common to all gas turbine engines form the power-producing part (known as the gas generator or core) and are, in the directio ...
engines included alloys like the
Nimonic Nimonic is a registered trademark of Special Metals Corporation that refers to a family of nickel-based high-temperature low creep superalloys. Nimonic alloys typically consist of more than 50% nickel and 20% chromium with additives such as titani ...
series alloys in the 1940s.
The early Nimonic series incorporated γ' Ni
3(Al,Ti)
precipitates
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading ...
in a γ matrix, as well as various metal-carbon
carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
s (e.g. Cr
23C
6) at the
grain boundaries
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional defects in the crystal structure, and tend to decrease the electrical and thermal ...
for additional grain boundary strength. Turbine blade components were
forged
Forging is a manufacturing process involving the shaping of metal using localized compressive forces. The blows are delivered with a hammer (often a power hammer) or a die. Forging is often classified according to the temperature at which it ...
until
vacuum induction casting
Casting is a manufacturing process in which a liquid material is usually poured into a mold, which contains a hollow cavity of the desired shape, and then allowed to solidify. The solidified part is also known as a ''casting'', which is ejected ...
technologies were introduced in the 1950s.
This process significantly improved cleanliness, reduced defects, and increased the strength and temperature capability of the material.
Modern superalloys were developed in the 1980s. The first generation superalloys incorporated increased
aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
,
titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
,
tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that ...
, and
niobium content in order to increase the γ' volume fraction in these alloys. Examples of first generation superalloys include: PWA1480, René N4 and SRR99. Additionally, the
volume fraction
In chemistry and fluid mechanics, the volume fraction φ''i'' is defined as the volume of a constituent ''V'i'' divided by the volume of all constituents of the mixture ''V'' prior to mixing:
:\phi_i = \frac
Being dimensionless, its unit is ...
of the γ' precipitates increased to about 50–70% with the advent of single crystal, or monocrystal, solidification techniques (see
Bridgman technique) for superalloys that enable grain boundaries to be entirely eliminated from a casting. Because the material contained no grain boundaries, carbides were unnecessary as grain boundary strengthers and were thus eliminated.
The second and third generation superalloys introduced about 3 and 6 weight per cent
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
, for increased temperature capability. Re is a slow diffuser and typically partitions to the γ matrix, decreasing the rate of diffusion (and thereby high temperature
creep) and improving high temperature performance and increasing service temperatures by 30 °C and 60 °C in second and third generation superalloys, respectively. Re has also been shown to promote the formation of rafts of the γ' phase (as opposed to cuboidal precipitates). The presence of rafts can decrease creep rate in the
power-law regime (controlled by dislocation climb), but can also potentially increase the creep rate if the dominant mechanism is particle shearing. Furthermore, Re tends to promote the formation of brittle
TCP phases, which has led to the strategy of reducing Co, W, Mo, and particularly Cr. Younger generations of Ni-based superalloys have significantly reduced Cr content for this reason, however with the reduction in Cr comes a reduction in
oxidation resistance. Advanced coating techniques are now used to offset the loss of
oxidation resistance accompanying the decreased Cr contents.
[Dunand, David C. "High-Temperature Materials for Energy Conversion" ''Materials Science & Engineering'' 381: Materials for Energy-Efficient Technology. Northwestern University, Evanston. 3 February 2015. Lecture.] Examples of second generation superalloys include PWA1484, CMSX-4 and René N5. Third generation alloys include CMSX-10, and René N6. Fourth, Fifth, and even Sixth generation superalloys have been developed which incorporate
ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
additions, making them more expensive still than the prior generation's Re-containing alloys. The effect of Ru on the promotion of TCP phases is not well-determined. Early reports determined that Ru decreased the supersaturation of Re in the matrix and thereby diminished the susceptibility to TCP phase formation.
[O'Hara, K. S., Walston, W. S., Ross, E. W., Darolia, R. US Patent 5482789, 1996.] More recent studies have noted the opposite effect. Chen, et al., found that in two alloys differing significantly only in Ru content (USTB-F3 and USTB-F6) that the addition of Ru increased both the partitioning ratio as well as the supersaturation in the γ matrix of Cr and Re, and thereby promoted the formation of TCP phases.
The current trend is to avoid very expensive and very heavy elements. An example is
Eglin steel Eglin steel (ES-1) is a high- strength, high-performance, low-alloy, low-cost steel, developed for a new generation of bunker buster type bombs, e.g. the Massive Ordnance Penetrator and the improved version of the GBU-28 bomb known as EGBU-28. It ...
, a budget material with compromised temperature range and chemical resistance. It does not contain rhenium or ruthenium and its nickel content is limited. To reduce fabrication costs, it was chemically designed to melt in a ladle (though with improved properties in a vacuum crucible). Also, conventional welding and casting is possible before heat-treatment. The original purpose was to produce high-performance, inexpensive bomb casings, but the material has proven widely applicable to structural applications, including armor.
Single-crystal superalloys
Single-crystal superalloys (SX or SC superalloys) are formed as a
single crystal
In materials science, a single crystal (or single-crystal solid or monocrystalline solid) is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries.RIWD. "Re ...
using a modified version of the directional solidification technique, so there are no
grain boundaries
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional defects in the crystal structure, and tend to decrease the electrical and thermal ...
in the material. The mechanical properties of most other alloys depend on the presence of grain boundaries, but at high temperatures, they would participate in
creep and must be replaced by other mechanisms. In many such alloys, islands of an ordered
intermetallic
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic eleme ...
phase sit in a matrix of disordered phase, all with the same crystalline lattice. This approximates the
dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to s ...
-pinning behavior of grain boundaries, without introducing any
amorphous solid
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wi ...
into the structure.
Single crystal (SX) superalloys have wide application in the high-pressure turbine section of aero and industrial gas turbine engines due to the unique combination of properties and performance. Since introduction of single crystal casting technology, SX alloy development has focused on increased temperature capability, and major improvements in alloy performance have been associated with the introduction of new alloying elements, including
rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
(Re) and
ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
(Ru).
With increasing turbine entry temperature, it is important to gain a fundamental understanding of the physical phenomena occurring during creep deformation of single crystal superalloys under such extreme condition (i.e. high temperature and high stress). The creep deformation behavior of superalloy single crystal is strongly temperature, stress, orientation and alloy dependent. For a single-crystal superalloy, there are 3 different modes of creep deformation under regimes of different temperature and stress: Rafting, Tertiary and Primary. At low temperature (~750 °C), SX alloys exhibits mostly primary creep behavior. Matan et al. concluded that the extent of primary creep deformation depends strongly on the angle between the tensile axis and the <001>/<011> symmetry boundary. At temperature above 850 °C, tertiary creep dominates and promotes strain softening behavior.
When temperature exceeds 1000 °C, the rafting effect is prevalent where cubic particles transform into flat shapes under tensile stress The rafts would also form perpendicular to the tensile axis, since γ phase was transported out of the vertical channels and into the horizontal ones. After conducting unaxial creep deformation of <001> orientated CMSX-4 single crystal superalloy at 1105 °C and 100 MPa, Reed et al. has established that rafting is beneficial to creep life since it delays evolution of creep strain. In addition, rafting would occur quickly and suppress the accumulation of creep strain until a critical strain is reached.
Oxidation in superalloys
For superalloys operating at high temperatures and exposed to
corrosive
A corrosive substance is one that will damage or destroy other substances with which it comes into contact by means of a chemical reaction.
Etymology
The word ''corrosive'' is derived from the Latin verb ''corrodere'', which means ''to gnaw'', ...
environments, the oxidation behavior is of paramount concern.
Oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
involves chemical reactions of the alloying elements with oxygen to form new
oxide phases, generally at the surface of the metal. If unmitigated, oxidation can degrade the alloy over time in a variety of ways, including:
* sequential oxidation, cracking, and
spalling of the surface, leading to erosion of the alloy over time
*
embrittlement
Embrittlement is a significant decrease of ductility of a material, which makes the material brittle. Embrittlement is used to describe any phenomena where the environment compromises a stressed material's mechanical performance, such as temperatu ...
of the surface through the introduction of oxide phases, promoting crack formation and
fatigue failure
*
depletion of key alloying elements, affecting the mechanical properties of the superalloy and possibly compromising its performance
The primary strategy used to limit these deleterious processes is called selective oxidation. Simply, the alloy is designed such that the ratio of alloying elements promotes formation of a specific oxide phase that can then act as a barrier to further oxidation. Most commonly,
aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
and
chromium are used in this role, because they form relatively thin and continuous oxide layers of
alumina (Al
2O
3) and
chromia
In Greek mythology, Chromia (; Ancient Greek: , ''Khrōmía'') was the daughter of Itonus, son of Amphictyon, himself son of Deucalion. She was also, in some traditions, the mother of Aetolus, Paeon, Epeius and Eurycyda by Endymion.
The poem ''E ...
(Cr
2O
3), respectively. Furthermore, they possess low oxygen
diffusivities, effectively halting further oxidation beneath this layer. In the ideal case, oxidation proceeds through two stages. First, transient oxidation involves the conversion of various elements, especially the majority elements (e.g. nickel or cobalt). Transient oxidation proceeds until the selective oxidation of the sacrificial element forms a complete barrier layer.
The protective effect of selective oxidation can be undermined by numerous mechanisms. The continuity of the thin sacrificial oxide layer can be compromised by mechanical disruption due to
stress
Stress may refer to:
Science and medicine
* Stress (biology), an organism's response to a stressor such as an environmental condition
* Stress (linguistics), relative emphasis or prominence given to a syllable in a word, or to a word in a phrase ...
or may be disrupted as a result of the
kinetics of oxidation (e.g. if diffusion of oxygen is too fast). If the layer is not continuous, its effectiveness as a diffusion barrier to oxygen is significantly reduced. The stability of the oxide layer is also strongly influenced by the presence of other minority elements. For example, the addition of
boron,
silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
, and
yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a " rare-earth element". Yttrium is almost always found in co ...
to superalloys promotes oxide layer
adhesion
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another ( cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another).
The forces that cause adhesion and cohesion can b ...
, reducing
spalling and maintaining the integrity of the protective oxide layer.
Oxidation is only the most basic form of chemical degradation superalloys may experience. More complex
corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
processes are common when operating environments include salts and sulfur compounds, or under chemical conditions that change dramatically over time. These issues and those of basic oxidation are often also addressed through thin coatings.
Superalloy processing
The historical developments in superalloy processing have brought about considerable increases in superalloy
operating temperatures. Superalloys were originally iron-based and cold wrought prior to the 1940s. In the 1940s
investment casting
Investment casting is an industrial process based on lost-wax casting, one of the oldest known metal-forming techniques. The term "lost-wax casting" can also refer to modern investment casting processes.
Investment casting has been used in var ...
of cobalt base alloys significantly raised operating temperatures. The development of
vacuum melting in the 1950s allowed for very fine control of the chemical composition of superalloys and reduction in contamination and in turn led to a revolution in processing techniques such as
directional solidification
Directional solidification (DS) and progressive solidification are types of solidification within castings. Directional solidification is solidification that occurs from farthest end of the casting and works its way towards the sprue. Progressiv ...
of alloys and single crystal superalloys.
There are many forms of superalloy present within a gas turbine engine, and processing methods vary widely depending on the necessary properties of each specific part.
Casting and forging
Casting and forging are traditional metallurgical processing techniques that can be used to generate both polycrystalline and monocrystalline products. Polycrystalline casts tend to have higher fracture resistance, while monocrystalline casts have higher creep resistance.
Jet turbine engines employ both poly and mono crystalline components to take advantage of their individual strengths. The disks of the high-pressure turbine, which are near the central hub of the engine are polycrystalline. The turbine blades, which extend radially into the engine housing, experience a much greater centripetal force, necessitating creep resistance. As a result, turbine blades are typically monocrystalline or polycrystalline with a preferred crystal orientation.
Investment casting
Investment casting
Investment casting is an industrial process based on lost-wax casting, one of the oldest known metal-forming techniques. The term "lost-wax casting" can also refer to modern investment casting processes.
Investment casting has been used in var ...
is a metallurgical processing technique in which a wax form is fabricated and used as a template for a ceramic mold. Briefly, a ceramic mold is poured around the wax form, the wax form is melted out of the ceramic mold, and molten metal is poured into the void left by the wax. This leads to a metal form in the same shape as the original wax form. Investment casting leads to a polycrystalline final product, as nucleation and growth of crystal grains occurs at numerous locations throughout the solid matrix. Generally, the polycrystalline product has no preferred grain orientation.
Directional solidification
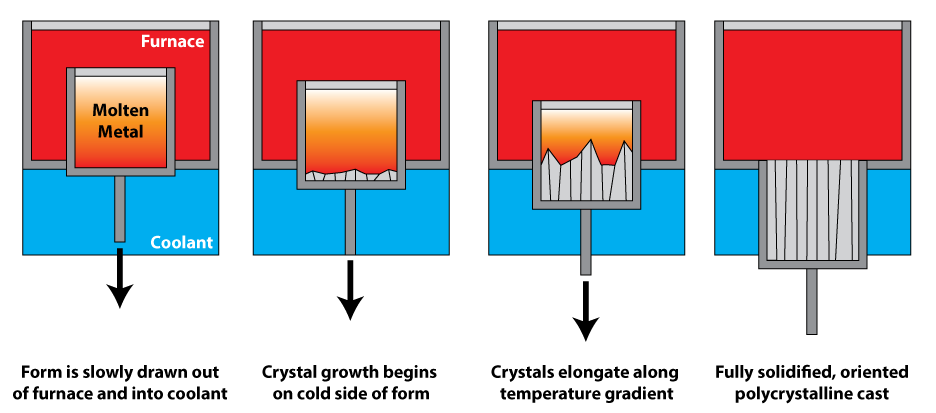
Directional solidification
Directional solidification (DS) and progressive solidification are types of solidification within castings. Directional solidification is solidification that occurs from farthest end of the casting and works its way towards the sprue. Progressiv ...
uses a thermal gradient to promote nucleation of metal grains on a low temperature surface, as well as to promote their growth along the temperature gradient. This leads to grains elongated along the temperature gradient, and significantly greater creep resistance parallel to the long grain direction. In polycrystalline turbine blades, directional solidification is used to orient the grains parallel to the centripetal force. It is also known as dendritic solidification.
Single crystal growth
Single crystal growth starts with a seed crystal which is used to template growth of a larger crystal. The overall process is lengthy, and additional processing via machining is necessary after the single crystal is grown.
Powder metallurgy
Powder metallurgy
Powder metallurgy (PM) is a term covering a wide range of ways in which materials or components are made from metal powders. PM processes can reduce or eliminate the need for subtractive processes in manufacturing, lowering material losses and ...
is a class of modern processing techniques in which metals are first converted into a powdered form, and then formed into the desired shape by heating below the melting point. This is in contrast to casting, which occurs with molten metal. Superalloy manufacturing often employs powder metallurgy because of its material efficiency - typically much less waste metal must be machined away from the final product—and its ability to facilitate mechanical alloying.
Mechanical alloying Mechanical alloying (MA) is a solid-state and powder processing technique involving repeated cold welding, fracturing, and re-welding of blended powder particles in a high-energy ball mill to produce a homogeneous material. Originally developed to ...
is a process by which reinforcing particles are incorporated into the superalloy matrix material by repeated fracture and welding.
Sintering and hot isostatic pressing
Sintering
Clinker nodules produced by sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction.
Sintering happens as part of a manufacturing ...
and
hot isostatic pressing
Hot isostatic pressing (HIP) is a manufacturing process, used to reduce the porosity of metals and increase the density of many ceramic materials. This improves the material's mechanical properties and workability.
The process can be used to pro ...
are processing techniques used to densify materials from a loosely packed "
green body
A green body is an object whose main constituent is weakly bound clay material, usually in the form of bonded powder or plates before it has been sintered or fired.
In ceramic engineering, the most common method for producing ceramic compon ...
" into a solid object with physically merged grains. Sintering occurs below the melting point, and causes adjacent particles to merge at their boundaries, leading to a strong bond between them. In hot isostatic pressing, a sintered material is placed in a pressure vessel and compressed from all directions (isostatically) in an inert atmosphere to affect densification.
Additive manufacturing
Selective laser melting
Selective laser melting (SLM) is one of many proprietary names for a metal additive manufacturing (AM) technology that uses a bed of powder with a source of heat to create metal parts. Also known as direct metal laser sintering (DMLS), the ASTM ...
(also known as ''powder bed fusion'') is an additive manufacturing procedure used to create intricately detailed forms from a CAD file. In CAD, a shape is designed and then converted into slices. These slices are sent to a laser writer to print the final product. In brief, a bed of metal powder is prepared, and the first slice of the CAD design is formed in the powder bed by a high energy laser sintering the particles together. After this first slice is generated, the powder bed moves downwards, and a new batch of metal powder is rolled over the top of the slice. The second layer is then sintered with the laser, and the process is repeated until all the slices in the CAD file have been processed. Due to the nature of many additive manufacturing processes, porosity can be present in products made by selective laser melting. Many products will often undergo a heat treatment or hot isostatic pressing procedure to densify the product and reduce porosity which can result in cracking.
Additive manufacturing for these applications is therefore particularly challenging.
Coating of superalloys
In modern gas turbine, the turbine entry temperature (~1750K) has exceeded the incipient melting temperature of superalloys (~1600K), with the help of surface engineering. Under such extreme working condition, the qualification of coating becomes vital.
Different types of coating
Historically, three "generations" of coatings have been developed: diffusion coatings, overlay coatings and thermal barrier coatings. Diffusion coatings, mainly constituted with aluminide or platinum-aluminide, is still the most common form of surface protection. To further enhance resistance to corrosion and oxidation, MCrAlX-based overlay coatings (M=Ni or Co, X=Y, Hf, Si) are deposited to surface of superalloys. Compared to diffusion coatings, overlay coatings are less dependent on the composition of the substrate, but also more expensive, since they must be carried out by air or vacuum plasma spraying (APS/VPS) or else electron beam physical vapour deposition (EB-PVD). Thermal barrier coatings provide by far the best enhancement in working temperature and coating life. It is estimated that modern TBC of thickness 300 μm, if used in conjunction with a hollow component and cooling air, has the potential to lower metal surface temperatures by a few hundred degrees.
Thermal barrier coatings
Thermal barrier coatings (TBCs) are used extensively on the surface of superalloy in both commercial and military gas turbine engines to increase component life and engine performance.
A coating of about 1-200 µm can reduce the temperature at the superalloy surface by up to 200K. TBCs are really a system of coatings consisting of a bond coat, a thermally grown oxide (TGO), and a thermally insulating ceramic top coat. In most applications, the bond coat is either a MCrAlY (where M=Ni or NiCo) or a Pt modified aluminide coating. A dense bond coat is required to provide protection of the superalloy substrate from oxidation and hot corrosion attack and to form an adherent, slow growing TGO on its surface. The TGO is formed by oxidation of the aluminum that is contained in the bond coat. The current (first generation) thermal insulation layer is composed of 7wt %
yttria-stabilized zirconia
Yttria-stabilized zirconia (YSZ) is a ceramic in which the cubic crystal structure of zirconium dioxide is made stable at room temperature by an addition of yttrium oxide. These oxides are commonly called "zirconia" ( Zr O2) and "yttria" ( Y2 O3 ...
(7YSZ) with a typical thickness of 100–300 µm. Yttria stabilized zirconia is used due to its low thermal conductivity (2.6W/mK for fully dense material), relatively high coefficient of thermal expansion, and good high temperature stability. The electron beam directed vapor deposition (EB-DVD) process used to apply the TBC to turbine airfoils produces a columnar microstructure with several levels of porosity. The porosity between the columns is critical to providing strain tolerance (via a very low in-plane modulus), as it would otherwise spall on thermal cycling due to thermal expansion mismatch with the superalloy substrate. The porosity within the columns reduces the thermal conductivity of the coating.
Bond coat
The bond coat adheres the thermal barrier coating to the superalloy substrate. Additionally, the bond coat provides oxidation protection and functions as a diffusion barrier against the motion of substrate atoms towards the environment.
There are five major types of bond coats, the aluminides, the platinum-aluminides, MCrAlY, cobalt-cermets, and nickel-chromium.
For the aluminide bond coatings, the final composition and structure of the coating depends on the composition of the substrate. Aluminides also lack ductility below 750 °C, and exhibit a limited by thermomechanical fatigue strength. The Pt-aluminides are very similar to the aluminide bond coats except for a layer of Pt (5—10 μm) deposited on the blade. The Pt is believed to aid in oxide adhesion and contributes to hot corrosion. The cost of Pt plating is justified by the increased blade life span. The MCrAlY is the latest generation of bond coat and does not strongly interact with the substrate. Normally applied by plasma spraying, MCrAlY coatings are secondary aluminum oxide formers. This means that the coatings form an outer layer of chromium oxide (chromia), and a secondary aluminum oxide (alumina) layer underneath. These oxide formations occur at high temperatures in the range of those that superalloys usually encounter. The chromia provides oxidation and hot-corrosion resistance. The alumina controls oxidation mechanisms by limiting oxide growth by self-passivating. The yttrium enhances the oxide adherence to the substrate, and limits the growth of grain boundaries (which can lead to flaking of the coating). Investigation indicates that addition of rhenium and tantalum increases oxidation resistance.
Cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
-cermet-based coatings consisting of materials such as
tungsten carbide
Tungsten carbide (chemical formula: WC) is a chemical compound (specifically, a carbide) containing equal parts of tungsten and carbon atoms. In its most basic form, tungsten carbide is a fine gray powder, but it can be pressed and formed into ...
/cobalt can be used due to excellent resistance to abrasion, corrosion, erosion, and heat. These
cermet
A cermet is a composite material composed of ceramic (cer) and metal (met) materials.
A cermet can combine attractive properties of both a ceramic, such as high temperature resistance and hardness, and those of a metal, such as the ability to und ...
coatings perform well in situations where temperature and oxidation damage are significant concerns, such as boilers. One of the unique advantages of cobalt cermet coatings is a minimal loss of coating mass over time, due to the strength of carbides within the mixture. Overall, cermet coatings are useful in situations where mechanical demands are equal to chemical demands for superalloys. Nickel-chromium coatings are used most frequently in boilers fed by
fossil fuels, electric
furnaces
A furnace is a structure in which heat is produced with the help of combustion.
Furnace may also refer to:
Appliances Buildings
* Furnace (central heating): a furnace , or a heater or boiler , used to generate heat for buildings
* Boiler, used t ...
, and waste incineration furnaces, where the danger of oxidizing agents and corrosive compounds in the vapor must be dealt with. The specific method of spray-coating depends on the composition of the coatings. Nickel-chromium coatings that also contain iron or aluminum perform much better (in terms of corrosion resistance) when they are sprayed and laser glazed, while pure nickel-chromium coatings perform better when thermally sprayed exclusively.
Process methods of coating
Superalloy products that are subjected to high working temperatures and corrosive atmosphere (such as high-pressure turbine region of jet engines) are coated with various kinds of
coating
A coating is a covering that is applied to the surface of an object, usually referred to as the substrate. The purpose of applying the coating may be decorative, functional, or both. Coatings may be applied as liquids, gases or solids e.g. Pow ...
. Several kinds of coating process are applied: pack cementation process, gas phase coating (both are a type of
chemical vapor deposition (CVD)),
thermal spraying
Thermal spraying techniques are coating processes in which melted (or heated) materials are sprayed onto a surface. The "feedstock" (coating precursor) is heated by electrical (plasma or arc) or chemical means (combustion flame).
Thermal sprayi ...
, and
physical vapor deposition
Physical vapor deposition (PVD), sometimes called physical vapor transport (PVT), describes a variety of vacuum deposition methods which can be used to produce thin films and coatings on substrates including metals, ceramics, glass, and polym ...
. In most cases, after the coating process near-surface regions of parts are enriched with aluminium, the matrix of the coating being
nickel aluminide Nickel aluminide typically refers to the one of the two most widely used compounds, Ni3Al or NiAl, however is generally any aluminide from the Ni-Al system. These alloys are widely used due to their corrosion resistance, low-density and easy product ...
.
Pack cementation process
Pack cementation is a widely used chemical vapor deposition technique which consists of immersing the components to be coated in a metal powder mixture and ammonium halide activators and sealing them in a retort. The entire apparatus is placed inside a furnace and heated in a protective atmosphere to a lower than normal temperature for diffusion to take place, due to the halide salts chemical reaction which causes a eutectic bond between the two metals. The new surface alloy that is formed due to thermal diffused ion migration has a metallurgical bond to the surface substrate and a true intermetallic layer found in the gamma layer of the new surface alloys.
The traditional pack consists of four components:
Substrate or parts- Ferrous and non-ferrous
Powdered alloy- (Ti and/or Al, Si and/or Zn, B and/ or Cr)
Halide salt activator- Ammonium halide salts
Relatively inert filler powder (Al2O3, SiO2, or SiC)
Temperatures below (750 °C)
This process includes but is not limited to:
Aluminizing
Chromizing
Siliconizing
Sherardizing
Boronizing
Titaniumizing
Pack Cementation has had a revival in the last 10 years as it is being combined with other chemical processes to lower the temperatures of metal combinations even further and give intermetallic properties to different alloy combinations for surface treatments.
Thermal spraying
Thermal spraying is a process of applying coatings by heating a feedstock of precursor material and spraying it on a surface. Different specific techniques are used depending on desired particle size, coat thickness, spray speed, desired area, etc. The coatings applied by thermal spraying of any kind, however, rely on adhesion to the surface. As a result, the surface of the superalloy must be cleaned and prepared, usually polished, before application of the thermal coating.
Plasma spraying
Of the various thermal spray methods, one of the more ideal and commonly used techniques for coating superalloys is
plasma spraying. This is due to the versatility of usable coatings, and the high-temperature performance of plasma-sprayed coatings. Plasma spraying can accommodate a very wide range of materials, much more so than other techniques. As long as the difference between melting and decomposition temperatures is greater than 300 Kelvin, a material can be melted and applied as a coating via plasma spraying.
Gas phase coating
This process is carried out at higher temperatures, about 1080 °C. The coating material is usually loaded onto special trays without physical contact with the parts to be coated. The coating mixture contains active coating material and activator, but usually does not contain thermal ballast. As in the pack cementation process, the gaseous aluminium chloride (or fluoride) is transferred to the surface of the part. However, in this case the diffusion is outwards. This kind of coating also requires diffusion heat treatment.
Failure mechanisms in thermal barrier coating systems
Failure of thermal barrier coating usually manifests as delamination, which arises from the temperature gradient during thermal cycling between ambient temperature and working conditions coupled with the difference in thermal expansion coefficient of the substrate and coating. It is rare for the coating to fail completely – some pieces of it remain intact, and significant scatter is observed in the time to failure if testing is repeated under identical conditions.
There are various degradation mechanisms for thermal barrier coating, and some or all of these must operate before failure finally occurs:
* Oxidation at the interface of thermal barrier coating and underlying bond coat;
* The depletion of aluminum in bond coat due to oxidation and diffusion with substrate;
* Thermal stresses from mismatch in thermal expansion coefficient and growth stress due to the formation of thermally grown oxide layer;
* Imperfections near thermally grown oxide layer;
* Various other complicating factors during engine operation.
Additionally, TBC life is very dependent upon the combination of materials (substrate, bond coat, ceramic) and processes (EB-PVD, plasma spraying) used.
Applications
Turbines
Nickel-based superalloys are used in load-bearing structures to the highest homologous temperature of any common alloy system (Tm = 0.9, or 90% of their melting point). Among the most demanding applications for a structural material are those in the hot sections of
turbine engines
A turbine ( or ) (from the Greek , ''tyrbē'', or Latin ''turbo'', meaning vortex) is a rotary mechanical device that extracts energy from a fluid flow and converts it into useful work. The work produced by a turbine can be used for generating e ...
(e.g.
). The preeminence of superalloys is reflected in the fact that they currently comprise over 50% of the weight of advanced aircraft engines. The widespread use of superalloys in turbine engines coupled with the fact that the thermodynamic efficiency of turbine engines is increased with increasing turbine inlet temperatures has, in part, provided the motivation for increasing the maximum-use temperature of superalloys. In fact, during the past 30 years turbine airfoil temperature capability has increased on average by about 4 °F (2.2 °C) per year. Two major factors which have made this increase possible are:
# Advanced processing techniques, which improved alloy cleanliness (thus improving reliability) and/or enabled the production of tailored microstructures such as directionally solidified or single-crystal material.
# Alloy development resulting in higher-use-temperature materials primarily through the additions of refractory elements such as Re, W, Ta, and Mo.
About 60% of the use-temperature increases have occurred due to advanced cooling concepts; 40% have resulted from material improvements. State-of-the-art turbine blade surface temperatures are near ; the most severe combinations of stress and temperature corresponds to an average bulk metal temperature approaching .
Although Nickel-based superalloys retain significant strength to temperatures near , they tend to be susceptible to environmental attack because of the presence of reactive alloying elements (which provide their high-temperature strength). Surface attack includes oxidation, hot corrosion, and thermal fatigue. In the most demanding applications, such as turbine blade and vanes, superalloys are often coated to improve environmental resistance.
In general, high temperature materials are needed for energy conversion and energy production applications. Maximum
energy conversion efficiency is desired in these energy applications, which can be achieved by increasing operating temperatures, as described by the Carnot cycle. Because Carnot efficiency is limited by the temperature difference between the hot and cold reservoirs, higher operating temperatures result in higher energy conversion efficiencies. Operating temperatures are limited by the performance of today’s superalloys, and currently, most applications operate at around 1000 °C-1400 °C. Energy applications and their superalloy components include:
* Gas turbines (turbine blades)
* Solar thermal power plants (stainless steel rods containing heated water)
* Steam turbines (turbine blades and boiler housing)
* Heat exchangers for nuclear reactor systems
Alumina-forming stainless steels can be processed via melting and
ladle casting, similar to the production of more common steels. Compared to
vacuum casting processes, ladle casting is much less expensive. In addition, alumina-forming stainless steel has been shown to be weldable and has potential for use in high performance automotive applications, such as for high temperature exhaust piping and in heat capture and reuse.
Research and development of new superalloys
The availability of superalloys during past decades has led to a steady increase in turbine entry temperatures, and the trend is expected to continue.
Sandia National Laboratories
Sandia National Laboratories (SNL), also known as Sandia, is one of three research and development laboratories of the United States Department of Energy's National Nuclear Security Administration (NNSA). Headquartered in Kirtland Air Force Bas ...
is studying a new method for making superalloys, known as
radiolysis. It introduces an entirely new area of research into creating alloys and superalloys through
nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
synthesis. This process holds promise as a universal method of
nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
formation. By developing an understanding of the basic
material science behind these nanoparticle formations, there is speculation that it might be possible to expand research into other aspects of superalloys.
There may be considerable disadvantages in making alloys by this method. About half of the use of superalloys is in applications where the service temperature is close to the melting temperature of the alloy. It is common therefore to use single crystals. The above method produces polycrystalline alloys, which suffer from an unacceptable level of creep.
Future paradigms in alloy development are expected to focus on weight reduction and improving oxidation and corrosion resistance while maintaining the strength of the alloy. Furthermore, with the increasing demand for turbine blades for power generation, another focus of alloy design is to reduce the cost of superalloys.
There has been ongoing research and development of new stainless steel alloys because of the lower costs in producing such alloys, as well as the need for an austenitic stainless steel with high-temperature corrosion resistance in environments with water vapor. Research is focusing on increasing high-temperature tensile strength, toughness, and creep resistance to compete with Ni-based superalloys.
A new class of alumina-forming austenitic stainless steel is actively being developed for use in high-temperature applications by Oak Ridge National Laboratory. Initial research showed similar creep and corrosion resistance at 800 °C to that of other austenitic alloys, including Ni-based superalloys.
Development of AFA superalloys with a 35 wt.% Ni-base have shown potential for use in operating temperatures upwards to 1,100 °C.
See also
*
Oxide dispersion strengthened alloy Oxide dispersion strengthened alloys (ODS) are alloys that consist of a metal matrix with small oxide particles dispersed within it. They have high heat resistance, strength, and ductility. Alloys of nickel are the most common but includes iron alum ...
*
Titanium aluminide
Titanium aluminide (chemical formula TiAl), commonly gamma titanium, is an intermetallic chemical compound. It is lightweight and resistant to oxidation and heat, but has low ductility. The density of γ-TiAl is about 4.0 g/cm3. It finds use in s ...
References
Bibliography
*
*
External links
* {{cite web , url=http://www.msm.cam.ac.uk/phase-trans/2003/nickel.html , title=Superalloys , publisher=Cambridge University Extensive bibliography and links.
Metallurgy
Aerospace materials
Emerging technologies
