Staurosporine on:
[Wikipedia]
[Google]
[Amazon]
Staurosporine (antibiotic AM-2282 or STS) is a


 This is followed by a nucleophilic attack between the indole nitrogens resulting in cyclization and then
This is followed by a nucleophilic attack between the indole nitrogens resulting in cyclization and then
natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical sy ...
originally isolated in 1977 from the bacterium '' Streptomyces staurosporeus''.
It was the first of over 50 alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of simila ...
s to be isolated with this type of bis-indole chemical structure. The chemical structure of staurosporine was elucidated by X-ray analysis of a single crystal and the absolute stereochemical configuration by the same method in 1994.
Staurosporine was discovered to have biological activities ranging from anti-fungal to anti-hypertensive.
The interest in these activities resulted in a large investigative effort in chemistry and biology and the discovery of the potential for anti-cancer treatment.
Biological activities
The main biological activity of staurosporine is the inhibition ofprotein kinase
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a fu ...
s through the prevention of ATP binding to the kinase. This is achieved through the stronger affinity of staurosporine to the ATP-binding site on the kinase. Staurosporine is a prototypical ATP-competitive kinase inhibitor in that it binds to many kinases with high affinity, though with little selectivity. Structural analysis of kinase pockets demonstrated that main chain atoms which are conserved in their relative positions to staurosporine contributes to staurosporine promiscuity. This lack of specificity has precluded its clinical use, but has made it a valuable research tool. In research, staurosporine is used to induce apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes ( morphology) and death. These changes in ...
. The mechanism of how it mediates this is not well understood. It has been found that one way in which staurosporine induces apoptosis is by activating caspase-3. At lower concentration, depending on the cell type, staurosporine induces specific cell cycle effects arresting cells either in G1 or in G2 phase of the cell cycle.
Chemistry family
Staurosporine is anindolocarbazole
Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range o ...
. It belongs to the most frequently isolated group of indolocarbazoles: Indolo(2,3-a)carbazoles. Of these, Staurosporine falls within the most common subgroup, called Indolo(2,3-a)pyrrole(3,4-c)carbazoles. These fall into two classes - halogenated (chlorinated) and non-halogenated. Halogenated indolo(2,3-a)pyrrole(3,4-c)carbazoles have a fully oxidized C-7 carbon with only one indole nitrogen containing a β-glycosidic bond, while non-halogenated indolo(2,3-a)pyrrole(3,4-c)carbazoles have both indole nitrogens glycosylated, and a fully reduced C-7 carbon. Staurosporine is in the non-halogenated class.
Staurosporine is the precursor of the novel protein kinase inhibitor midostaurin
Midostaurin, sold under the brand name Rydapt & Tauritmo both by Novartis, is a multi-targeted protein kinase inhibitor that has been investigated for the treatment of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and advanced sys ...
(PKC412). Besides midostaurin, staurosporine is also used as a starting material in the commercial synthesis of K252c (also called staurosporine aglycone). In the natural biosynthetic pathway, K252c is a precursor of staurosporine.

Biosynthesis
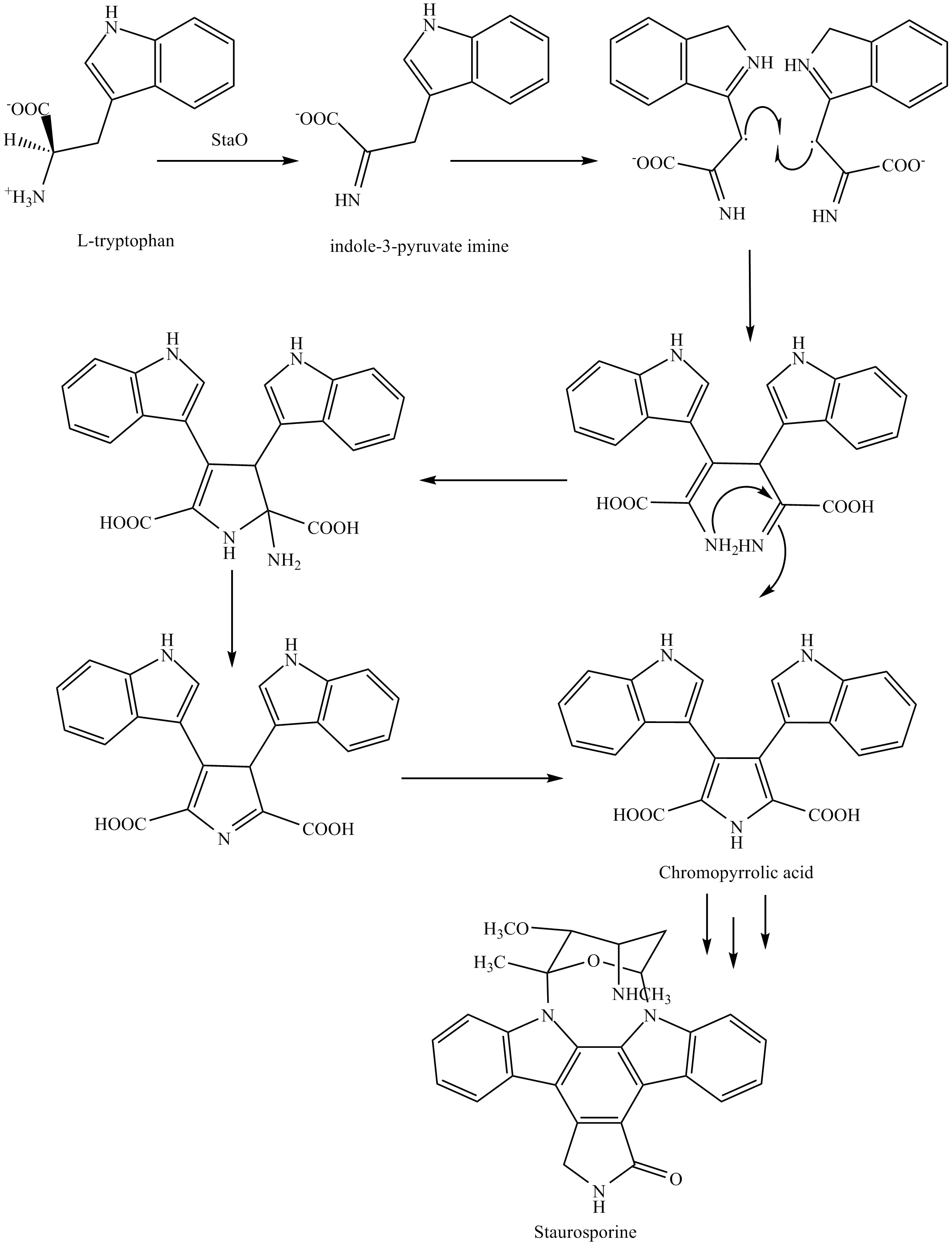
The biosynthesis of staurosporine starts with the amino acidL-tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
in its zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
ic form. Tryptophan is converted to an imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
by enzyme StaO which is an L-amino acid oxidase (that may be FAD dependent). The imine is acted upon by StaD to form an uncharacterized intermediate proposed to be the dimerization product between 2 imine molecules. Chromopyrrolic acid is the molecule formed from this intermediate after the loss of VioE (used in the biosynthesis of violacein – a natural product formed from a branch point in this pathway that also diverges to form rebeccamycin. An aryl aryl coupling thought to be catalyzed by a cytochrome P450 enzyme to form an aromatic ring system occurs.
 This is followed by a nucleophilic attack between the indole nitrogens resulting in cyclization and then
This is followed by a nucleophilic attack between the indole nitrogens resulting in cyclization and then decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
assisted by StaC exclusively forming staurosporine aglycone or K252c. Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
is transformed to NTP-L-ristoamine by StaA/B/E/J/I/K which is then added on to the staurosporine aglycone at 1 indole N by StaG. The StaN enzyme reorients the sugar by attaching it to the 2nd indole nitrogen into an unfavored conformation to form intermediated O-demethyl-N-demethyl-staurosporine. Lastly, O-methylation of the 4'amine by StaMA and N-methylation of the 3'-hydroxy by StaMB leads to the formation of staurosporine.
Research in preclinical use
When encapsulated inliposome
A liposome is a small artificial Vesicle (biology and chemistry), vesicle, spherical in shape, having at least one lipid bilayer. Due to their hydrophobicity and/or hydrophilicity, biocompatibility, particle size and many other properties, lipo ...
nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
, staurosporine is shown to suppress tumors ''in vivo'' in a mouse model without the toxic side effects which have prohibited its use as an anti-cancer drug with high apoptotic activity. Researchers in UC San Diego Moores Cancer Center
The Rebecca and John Moores Cancer Center is the region's only NCI-designated Cancer Center in La Jolla, California, part of UC San Diego Health and affiliated with the University of California San Diego. It is supported, in part, by the National ...
develop a platform technology of high drug-loading efficiency by manipulating the pH environment of the cells. When injected into the mouse glioblastoma
Glioblastoma, previously known as glioblastoma multiforme (GBM), is one of the most aggressive types of cancer that begin within the brain. Initially, signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality ...
model, staurosporine is found to accumulate primarily in the tumor via fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
confirmation, and the mice did not suffer weight loss compared to the control mice administered with the free compound, an indicator of reduced toxicity.
List of compounds closely related to Staurosporine
*K252a
K252a is an alkaloid isolated from '' Nocardiopsis'' bacteria. This staurosporine analog is a highly potent cell permeable inhibitor of CaM kinase and phosphorylase kinase (IC50 = 1.8 and 1.7 nmol/ L, respectively). At higher concentrations it ...
* Stauprimide
Stauprimide is a semi-synthetic analog of the staurosporine family of indolocarbazoles. Stauprimide was first published in 1994 as part of an extensive structure-activity investigation to improve the selective inhibition of protein kinase C as a ...
* Midostaurin
Midostaurin, sold under the brand name Rydapt & Tauritmo both by Novartis, is a multi-targeted protein kinase inhibitor that has been investigated for the treatment of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and advanced sys ...
References
{{reflist Bacterial alkaloids Antibiotics Gamma-lactams Protein kinase inhibitors Indolocarbazoles