Stability constants of complexes on:
[Wikipedia]
[Google]
[Amazon]
In
A comprehensive database of published data on equilibrium constants of metal complexes and ligandsNIST Standard Reference Database 46
NIST Critically Selected Stability Constants of Metal Complexes: Version 8.0 (This database has been discontinued.)
+ 2L <=> ML2 ;
The stepwise constants, ''K''1 and ''K''2 refer to the formation of the complexes one step at a time.
: + L <=> ML ;
: + L <=> ML2 ;
It follows that
:
A cumulative constant can always be expressed as the product of stepwise constants. Conversely, any stepwise constant can be expressed as a quotient of two or more overall constants. There is no agreed notation for stepwise constants, though a symbol such as ''K'' is sometimes found in the literature. It is good practice to specify each stability constant explicitly, as illustrated above.
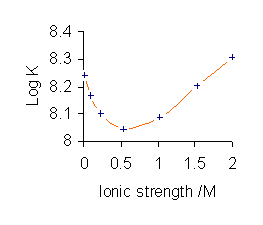 To avoid the complications involved in using activities, stability constants are determined, where possible, in a medium consisting of a solution of a background
To avoid the complications involved in using activities, stability constants are determined, where possible, in a medium consisting of a solution of a background
 Consider the two equilibria, in aqueous solution, between the
Consider the two equilibria, in aqueous solution, between the  These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is favourable in this instance. In general it is difficult to account precisely for thermodynamic values in terms of changes in solution at the molecular level, but it is clear that the chelate effect is predominantly an effect of entropy. Other explanations, including that of Schwarzenbach, are discussed in Greenwood and Earnshaw.
The chelate effect increases as the number of chelate rings increases. For example, the complex i(dien)2)sup>2+ is more stable than the complex i(en)3)sup>2+; both complexes are octahedral with six nitrogen atoms around the nickel ion, but dien (
These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is favourable in this instance. In general it is difficult to account precisely for thermodynamic values in terms of changes in solution at the molecular level, but it is clear that the chelate effect is predominantly an effect of entropy. Other explanations, including that of Schwarzenbach, are discussed in Greenwood and Earnshaw.
The chelate effect increases as the number of chelate rings increases. For example, the complex i(dien)2)sup>2+ is more stable than the complex i(en)3)sup>2+; both complexes are octahedral with six nitrogen atoms around the nickel ion, but dien (
HYPERQUAD
BEST
ReactLab pH PRO
* Spectrophotometric data
SQUAD, SPECFIT
ReactLab EQUILIBRIA
* NMR dat
In biochemistry, formation constants of adducts may be obtained from
Stability constants website
Contains information on computer programs, applications, databases and hardware for experimental titrations. {{Chemical equilibria Equilibrium chemistry Coordination chemistry
coordination chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Man ...
, a stability constant (also called formation constant or binding constant) is an equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.
History
Jannik Bjerrum (son of Niels Bjerrum) developed the first general method for the determination of stability constants of metal-ammine complexes in 1941. The reasons why this occurred at such a late date, nearly 50 years afterAlfred Werner
Alfred Werner (12 December 1866 – 15 November 1919) was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration ...
had proposed the correct structures for coordination complexes, have been summarised by Beck and Nagypál. The key to Bjerrum's method was the use of the then recently developed glass electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. The most common application of ion-selective glass electrodes is for the measurement of pH. The pH electrode is an exampl ...
and pH meter
A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH elect ...
to determine the concentration of hydrogen ions
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle ...
in solution. Bjerrum recognised that the formation of a metal complex with a ligand was a kind of acid–base equilibrium: there is competition for the ligand, L, between the metal ion, Mn+, and the hydrogen ion, H+. This means that there are two simultaneous equilibria that have to be considered. In what follows electrical charges are omitted for the sake of generality. The two equilibria are
:
:
Hence by following the hydrogen ion concentration during a titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' ...
of a mixture of M and HL with base, and knowing the acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:HA ...
of HL, the stability constant for the formation of ML could be determined. Bjerrum went on to determine the stability constants for systems in which many complexes may be formed.
:
The following twenty years saw a veritable explosion in the number of stability constants that were determined. Relationships, such as the Irving-Williams series were discovered. The calculations were done by hand using the so-called graphical methods. The mathematics underlying the methods used in this period are summarised by Rossotti and Rossotti. The next key development was the use of a computer program, LETAGROP to do the calculations. This permitted the examination of systems too complicated to be evaluated by means of hand-calculations. Subsequently, computer programs capable of handling complex equilibria in general, such as SCOGS and MINIQUAD were developed so that today the determination of stability constants has almost become a "routine" operation. Values of thousands of stability constants can be found in two commercial databases.IUPAC SC-DatabaseA comprehensive database of published data on equilibrium constants of metal complexes and ligandsNIST Standard Reference Database 46
NIST Critically Selected Stability Constants of Metal Complexes: Version 8.0 (This database has been discontinued.)
Theory
The formation of a complex between a metal ion, M, and a ligand, L, is in fact usually a substitution reaction. For example, inaqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be r ...
s, metal ions will be present as aqua ions, so the reaction for the formation of the first complex could be written as
:
The equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
for this reaction is given by
:
should be read as "the concentration of L" and likewise for the other terms in square brackets. The expression can be greatly simplified by removing those terms which are constant. The number of water molecules attached to each metal ion is constant. In dilute solutions the concentration of water is effectively constant. The expression becomes
:
Following this simplification a general definition can be given, for the general equilibrium
:
:
The definition can easily be extended to include any number of reagents. The reagents need not always be a metal and a ligand but can be any species which form a complex. Stability constants defined in this way, are ''association'' constants. This can lead to some confusion as p''K''a values are ''dissociation'' constants. In general purpose computer programs it is customary to define all constants as association constants. The relationship between the two types of constant is given in association and dissociation constants.
Stepwise and cumulative constants
A cumulative or overall constant, given the symbol ''β'', is the constant for the formation of a complex from reagents. For example, the cumulative constant for the formation of ML2 is given by :Hydrolysis products
The formation of a hydroxo complex is a typical example of a hydrolysis reaction. A hydrolysis reaction is one in which a substrate reacts with water, splitting a water molecule into hydroxide and hydrogen ions. In this case the hydroxide ion then forms a complex with the substrate. :; In water the concentration ofhydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. ...
is related to the concentration of hydrogen ions by the self-ionization constant
The self-ionization of water (also autoionization of water, and autodissociation of water) is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H2O, deprotonates (loses the nucleus of one of its hydrogen ...
, ''K''w.
:
The expression for hydroxide concentration is substituted into the formation constant expression
:
:
In general, for the reaction
:
In the older literature the value of log K is usually cited for an hydrolysis constant. The log ''β''* value is usually cited for an hydrolysed complex with the generic chemical formula MpLq(OH)r.
Acid–base complexes
ALewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, A, and a Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, B, can be considered to form a complex AB.
:
There are three major theories relating to the strength of Lewis acids and bases and the interactions between them.
#Hard and soft acid–base theory (HSAB
HSAB concept is a jargon for "hard and soft (Lewis) acids and bases". HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemic ...
). This is used mainly for qualitative purposes.
#Drago and Wayland proposed a two-parameter equation which predicts the standard enthalpy of formation of a very large number of adducts quite accurately. −Δ''H''⊖ (A − B) = ''E''A''E''B + ''C''A''C''B. Values of the ''E'' and ''C'' parameters are available.
#Guttmann donor number In chemistry a donor number (DN) is a quantitative measure of Lewis basicity. A donor number is defined as the negative enthalpy value for the 1:1 adduct formation between a Lewis base and the standard Lewis acid SbCl5 (antimony pentachloride), in ...
s: for bases the number is derived from the enthalpy of reaction of the base with antimony pentachloride
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substa ...
in 1,2-Dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vin ...
as solvent. For acids, an acceptor number is derived from the enthalpy of reaction of the acid with triphenylphosphine oxide
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). This colourless crystalline compound is a common but potentially useful waste product in ...
.
For more details see: acid–base reaction
An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their appl ...
, acid catalysis
In acid catalysis and base catalysis, a chemical reaction is catalyzed by an acid or a base. By Brønsted–Lowry acid–base theory, the acid is the proton (hydrogen ion, H+) donor and the base is the proton acceptor. Typical reactions catalyze ...
, Extraction (chemistry)
Extraction in chemistry is a separation process consisting of the separation of a substance from a matrix. Common examples include '' liquid-liquid extraction'', and ''solid phase extraction''. The distribution of a solute between two phases ...
Thermodynamics
The thermodynamics of metal ion complex formation provides much significant information. In particular it is useful in distinguishing betweenenthalpic
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
and entropic
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
effects. Enthalpic effects depend on bond strengths and entropic effects have to do with changes in the order/disorder of the solution as a whole. The chelate effect, below, is best explained in terms of thermodynamics.
An equilibrium constant is related to the standard Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature an ...
change for the reaction
:
''R'' is the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
and ''T'' is the absolute temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic ...
. At 25 °C, . Free energy is made up of an enthalpy term and an entropy term.
:
The standard enthalpy change can be determined by calorimetry
In chemistry and thermodynamics, calorimetry () is the science or act of measuring changes in ''state variables'' of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical re ...
or by using the Van 't Hoff equation
The Van 't Hoff equation relates the change in the equilibrium constant, , of a chemical reaction to the change in temperature, ''T'', given the standard enthalpy change, , for the process. It was proposed by Dutch chemist Jacobus Henricus van ' ...
, though the calorimetric method is preferable. When both the standard enthalpy change and stability constant have been determined, the standard entropy change is easily calculated from the equation above.
The fact that stepwise formation constants of complexes of the type ML''n'' decrease in magnitude as ''n'' increases may be partly explained in terms of the entropy factor. Take the case of the formation of octahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet a ...
complexes.
:
For the first step ''m'' = 6, ''n'' = 1 and the ligand can go into one of 6 sites. For the second step ''m'' = 5 and the second ligand can go into one of only 5 sites. This means that there is more randomness in the first step than the second one; Δ''S''⊖ is more positive, so Δ''G''⊖ is more negative and . The ratio of the stepwise stability constants can be calculated on this basis, but experimental ratios are not exactly the same because Δ''H''⊖ is not necessarily the same for each step. Exceptions to this rule are discussed below, in #chelate effect and #Geometrical factors.
Ionic strength dependence
The thermodynamic equilibrium constant, ''K''⊖, for the equilibrium : can be defined as : where is the activity of the chemical species ML etc. ''K''⊖ isdimensionless
A dimensionless quantity (also known as a bare quantity, pure quantity, or scalar quantity as well as quantity of dimension one) is a quantity to which no physical dimension is assigned, with a corresponding SI unit of measurement of one (or 1) ...
since activity is dimensionless. Activities of the products are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same ...
for a derivation of this expression.
Since activity is the product of concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', ''number concentration'', ...
and activity coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same ...
(''γ'') the definition could also be written as
:
where Lrepresents the concentration of ML and ''Γ'' is a quotient of activity coefficients. This expression can be generalized as
:
 To avoid the complications involved in using activities, stability constants are determined, where possible, in a medium consisting of a solution of a background
To avoid the complications involved in using activities, stability constants are determined, where possible, in a medium consisting of a solution of a background electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon ...
at high ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such a ...
, that is, under conditions in which ''Γ'' can be assumed to be always constant. For example, the medium might be a solution of 0.1 mol dm−3 sodium nitrate
Sodium nitrate is the chemical compound with the formula . This alkali metal nitrate salt is also known as Chile saltpeter (large deposits of which were historically mined in Chile) to distinguish it from ordinary saltpeter, potassium nitrate. ...
or 3 mol dm−3 sodium perchlorate
Sodium perchlorate is the inorganic compound with the chemical formula Na ClO4. It is a white crystalline, hygroscopic solid that is highly soluble in water and in alcohol. It is usually encountered as the monohydrate. The compound is noteworthy ...
. When ''Γ'' is constant it may be ignored and the general expression in theory, above, is obtained.
All published stability constant values refer to the specific ionic medium used in their determination and different values are obtained with different conditions, as illustrated for the complex CuL (L = glycinate
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
). Furthermore, stability constant values depend on the specific electrolyte used as the value of ''Γ'' is different for different electrolytes, even at the same ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such a ...
. There does not need to be any chemical interaction between the species in equilibrium and the background electrolyte, but such interactions might occur in particular cases. For example, phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
s form weak complexes with alkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
, so, when determining stability constants involving phosphates, such as ATP, the background electrolyte used will be, for example, a tetralkylammonium salt. Another example involves iron(III) which forms weak complexes with halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a flu ...
and other anions, but not with perchlorate
A perchlorate is a chemical compound containing the perchlorate ion, . The majority of perchlorates are commercially produced salts. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. ...
ions.
When published constants refer to an ionic strength other than the one required for a particular application, they may be adjusted by means of specific ion theory (SIT) and other theories.
Temperature dependence
All equilibrium constants vary withtemperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
according to the Van 't Hoff equation
The Van 't Hoff equation relates the change in the equilibrium constant, , of a chemical reaction to the change in temperature, ''T'', given the standard enthalpy change, , for the process. It was proposed by Dutch chemist Jacobus Henricus van ' ...
:
Alternatively
:
''R'' is the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
and ''T'' is the thermodynamic temperature. Thus, for exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ...
reactions, where the standard enthalpy change, Δ''H''⊖, is negative, ''K'' decreases with temperature, but for endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
reactions, where Δ''H''⊖ is positive, ''K'' increases with temperature.
Factors affecting the stability constants of complexes
The chelate effect
copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
(II) ion, Cu2+ and ethylenediamine
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately ...
(en) on the one hand and methylamine
Methylamine is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.
Methylamine is sold as a solution in methanol, ...
, MeNH2 on the other.
:
:
In the first reaction the bidentate ligand ethylene diamine forms a chelate complex with the copper ion. Chelation results in the formation of a five-membered ring. In the second reaction the bidentate ligand is replaced by two monodentate
In coordination chemistry, denticity () refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals ...
methylamine ligands of approximately the same donor power, meaning that the enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
of formation of Cu–N bonds is approximately the same in the two reactions. Under conditions of equal copper concentrations and when then concentration of methylamine is twice the concentration of ethylenediamine, the concentration of the bidentate complex will be greater than the concentration of the complex with 2 monodentate ligands. The effect increases with the number of chelate rings so the concentration of the EDTA
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula H2N(CH2CO2H)2sub>2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes ev ...
complex, which has six chelate rings, is much higher than a corresponding complex with two monodentate nitrogen donor ligands and four monodentate carboxylate ligands. Thus, the phenomenon
A phenomenon ( : phenomena) is an observable event. The term came into its modern philosophical usage through Immanuel Kant, who contrasted it with the noumenon, which ''cannot'' be directly observed. Kant was heavily influenced by Gottfrie ...
of the chelate effect is a firmly established empirical
Empirical evidence for a proposition is evidence, i.e. what supports or counters this proposition, that is constituted by or accessible to sense experience or experimental procedure. Empirical evidence is of central importance to the sciences and ...
fact: under comparable conditions, the concentration of a chelate complex will be higher than the concentration of an analogous complex with monodentate ligands.
The thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of ...
approach to explaining the chelate effect considers the equilibrium constant for the reaction: the larger the equilibrium constant, the higher the concentration of the complex.
:
:
When the analytical concentration
Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of solut ...
of methylamine is twice that of ethylenediamine and the concentration of copper is the same in both reactions, the concentration u(en)sup>2+ is much higher than the concentration u(MeNH2)2sup>2+ because
The difference between the two stability constants is mainly due to the difference in the standard entropy change, Δ''S''⊖. In the reaction with the chelating ligand there are two particles on the left and one on the right, whereas in equation with the monodentate ligand there are three particles on the left and one on the right. This means that less entropy of disorder is lost when the chelate complex is formed than when the complex with monodentate ligands is formed. This is one of the factors contributing to the entropy difference. Other factors include solvation changes and ring formation. Some experimental data to illustrate the effect are shown in the following table. p. 910
:
diethylenetriamine
Diethylenetriamine (abbreviated and also known as 2,2’-Iminodi(ethylamine)) is an organic compound with the formula HN(CH2CH2NH2)2. This colourless hygroscopic liquid is soluble in water and polar organic solvents, but not simple hydrocarbon ...
, 1,4,7-triazaheptane) is a tridentate ligand and en is bidentate. The number of chelate rings is one less than the number of donor atoms in the ligand. EDTA
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula H2N(CH2CO2H)2sub>2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes ev ...
(ethylenediaminetetracetic acid) has six donor atoms so it forms very strong complexes with five chelate rings. Ligands such as DTPA, which have eight donor atoms are used to form complexes with large metal ions such as lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
or actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
ions which usually form 8- or 9-coordinate complexes.
5-membered and 6-membered chelate rings give the most stable complexes. 4-membered rings are subject to internal strain because of the small inter-bond angle is the ring. The chelate effect is also reduced with 7- and 8- membered rings, because the larger rings are less rigid, so less entropy is lost in forming them.
Deprotonation of aliphatic –OH groups
left, 125px, 2-aminoethanol 125px, Tris Removal of aproton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
from an aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane ...
–OH group is difficult to achieve in aqueous solution because the energy required for this process is rather large. Thus, ionization of aliphatic –OH groups occurs in aqueous solution only in special circumstances. One such circumstance is found with compounds containing the H2N–C–C–OH substructure. For example, compounds containing the 2-aminoethanol
Ethanolamine (2-aminoethanol, monoethanolamine, ETA, or MEA) is an organic chemical compound with the formula or . The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid ...
substructure can form metal–chelate complexes with the deprotonated form, H2N–C–C–O−. The chelate effect supplies the extra energy needed to break the O–H bond.
An important example occurs with the molecule tris
Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2, one of the twenty Good's buffers. It is extensively used in biochemistry and molecular biology as ...
. This molecule should be used with caution as a buffering agent
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is ...
as it will form chelate complexes with ions such as Fe3+ and Cu2+.
The macrocyclic effect
It was found that the stability of the complex of copper(II) with themacrocyclic
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
ligand cyclam
Cyclam (1,4,8,11-tetraazacyclotetradecane) is an organic compound with the formula (NHCH2CH2NHCH2CH2CH2)2. Classified as an aza-crown ether, it is a white solid that is soluble in water. As a macrocyclic ligand, it binds strongly to many transit ...
(1,4,8,11-tetraazacyclotetradecane) was much greater than expected in comparison to the stability of the complex with the corresponding open-chain amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
.
This phenomenon was named the macrocyclic effect and it was also interpreted as an entropy effect. However, later studies suggested that both enthalpy and entropy factors were involved.
An important difference between macrocyclic ligands and open-chain (chelating) ligands is that they have selectivity for metal ions, based on the size of the cavity into which the metal ion is inserted when a complex is formed. For example, the crown ether
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . Impo ...
18-crown-6
18-Crown-6 is an organic compound with the formula 2H4Osub>6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ...
forms much stronger complexes with the potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmos ...
ion, K+ than with the smaller sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
ion, Na+.
In hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythroc ...
an iron(II) ion is complexed by a macrocyclic porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical com ...
ring. The article hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythroc ...
incorrectly states that oxyhemoglogin contains iron(III). It is now known that the iron(II) in hemoglobin is a low-spin complex, whereas in oxyhemoglobin it is a high-spin complex. The low-spin Fe2+ ion fits snugly into the cavity of the porphyrin ring, but high-spin iron(II) is significantly larger and the iron atom is forced out of the plane of the macrocyclic ligand. This effect contributes the ability of hemoglobin to bind oxygen reversibly under biological conditions. In Vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. It is one of eight B vitamins. It is required by animals, which use it as a cofactor in DNA synthesis, in both fatty acid and amino acid metabolism. ...
a cobalt(II) ion is held in a corrin ring. Chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to ...
is a macrocyclic complex of magnesium(II).
Geometrical factors
Successive stepwise formation constants ''Kn'' in a series such as ML''n'' (''n'' = 1, 2, ...) usually decrease as ''n'' increases. Exceptions to this rule occur when the geometry of the ML''n'' complexes is not the same for all members of the series. The classic example is the formation of the diamminesilver(I) complex g(NH3)2sup>+ in aqueous solution. : : In this case, ''K''2 > ''K''1. The reason for this is that, in aqueous solution, the ion written as Ag+ actually exists as the four-coordinate tetrahedral aqua species g(H2O)4sup>+. The first step is then a substitution reaction involving the displacement of a bound water molecule by ammonia forming the tetrahedral complex g(NH3)(H2O)3sup>+. In the second step, all the aqua ligands are lost and a linear, two-coordinate product 3N–Ag–NH3sup>+ is formed. Examination of the thermodynamic data shows that the difference in entropy change is the main contributor to the difference in stability constants for the two complexation reactions. Other examples exist where the change is from octahedral to tetrahedral, as in the formation of oCl4sup>2− from o(H2O)6sup>2+.Classification of metal ions
Ahrland, Chatt and Davies proposed that metal ions could be described as class A if they formed stronger complexes with ligands whose donor atoms arenitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
or fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactiv ...
than with ligands whose donor atoms are phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
, sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
or chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
and class B if the reverse is true. For example, Ni2+ forms stronger complexes with amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
s than with phosphines
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
, but Pd2+ forms stronger complexes with phosphines than with amines. Later, Pearson proposed the theory of hard and soft acids and bases
HSAB concept is a jargon for "hard and soft (Lewis) acids and bases". HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chem ...
(HSAB theory). In this classification, class A metals are hard acids and class B metals are soft acids. Some ions, such as copper(I), are classed as borderline. Hard acids form stronger complexes with hard bases than with soft bases. In general terms hard–hard interactions are predominantly electrostatic in nature whereas soft–soft interactions are predominantly covalent in nature. The HSAB theory, though useful, is only semi-quantitative.
The hardness of a metal ion increases with oxidation state. An example of this effect is given by the fact that Fe2+ tends to form stronger complexes with ''N''-donor ligands than with ''O''-donor ligands, but the opposite is true for Fe3+.
Effect of ionic radius
The Irving–Williams series refers to high-spin, octahedral, divalent metal ion of the first transition series. It places the stabilities of complexes in the order :Mn < Fe < Co < Ni < Cu > Zn This order was found to hold for a wide variety of ligands. There are three strands to the explanation of the series. #Theionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the catio ...
is expected to decrease regularly for Mn2+ to Zn2+. This would be the normal periodic trend and would account for the general increase in stability.
#The crystal field stabilisation energy (CFSE) increases from zero for manganese(II) to a maximum at nickel(II). This makes the complexes increasingly stable. CFSE returns to zero for zinc(II).
#Although the CFSE for copper(II) is less than for nickel(II), octahedral copper(II) complexes are subject to the Jahn–Teller effect
The Jahn–Teller effect (JT effect or JTE) is an important mechanism of spontaneous symmetry breaking in molecular and solid-state systems which has far-reaching consequences in different fields, and is responsible for a variety of phenomena in sp ...
which results in a complex having extra stability.
Another example of the effect of ionic radius the steady increase in stability of complexes with a given ligand along the series of trivalent lanthanide ions, an effect of the well-known lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radii ...
.
Applications
Stability constant values are exploited in a wide variety of applications.Chelation therapy
Chelation therapy is a medical procedure that involves the administration of chelating agents to remove heavy metals from the body. Chelation therapy has a long history of use in clinical toxicology and remains in use for some very specific me ...
is used in the treatment of various metal-related illnesses, such as iron overload in β-thalassemia
Thalassemias are inherited blood disorders characterized by decreased hemoglobin production. Symptoms depend on the type and can vary from none to severe. Often there is mild to severe anemia (low red blood cells or hemoglobin). Anemia can resul ...
sufferers who have been given blood transfusions. The ideal ligand binds to the target metal ion and not to others, but this degree of selectivity is very hard to achieve. The synthetic drug deferiprone achieves selectivity by having two oxygen donor atoms so that it binds to Fe3+ in preference to any of the other divalent ions that are present in the human body, such as Mg2+, Ca2+ and Zn2+. Treatment of poisoning by ions such as Pb2+ and Cd2+ is much more difficult since these are both divalent ions and selectivity is harder to accomplish. Excess copper in Wilson's disease
Wilson's disease is a genetic disorder in which excess copper builds up in the body. Symptoms are typically related to the brain and liver. Liver-related symptoms include vomiting, weakness, fluid build up in the abdomen, swelling of the legs, ...
can be removed by penicillamine
Penicillamine, sold under the brand name of Cuprimine among others, is a medication primarily used for the treatment of Wilson's disease. It is also used for people with kidney stones who have high urine cystine levels, rheumatoid arthritis, ...
or Triethylene tetramine (TETA). DTPA has been approved by the U.S. Food and Drug Administration for treatment of plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
poisoning.
DTPA is also used as a complexing agent for gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen ...
in MRI contrast enhancement
A contrast agent (or contrast medium) is a substance used to increase the contrast of structures or fluids within the body in medical imaging. Contrast agents absorb or alter external electromagnetism or ultrasound, which is different from radiop ...
. The requirement in this case is that the complex be very strong, as Gd3+ is very toxic. The large stability constant of the octadentate ligand ensures that the concentration of free Gd3+ is almost negligible, certainly well below toxicity threshold. In addition the ligand occupies only 8 of the 9 coordination sites on the gadolinium ion. The ninth site is occupied by a water molecule which exchanges rapidly with the fluid surrounding it and it is this mechanism that makes the paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
complex into a contrast reagent.
EDTA
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula H2N(CH2CO2H)2sub>2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes ev ...
forms such strong complexes with most divalent cations that it finds many uses
Use may refer to:
* Use (law), an obligation on a person to whom property has been conveyed
* Use (liturgy), a special form of Roman Catholic ritual adopted for use in a particular diocese
* Use–mention distinction, the distinction between using ...
. For example, it is often present in washing powder to act as a water softener by sequestering calcium and magnesium ions.
The selectivity of macrocyclic ligands can be used as a basis for the construction of an ion selective electrode. For example, potassium selective electrodes are available that make use of the naturally occurring macrocyclic antibiotic valinomycin.
An ion-exchange resin
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radius) microbeads, usually white or ye ...
such as chelex 100 Chelex 100 is a chelating material from Bio-Rad used to purify other compounds via ion exchange. It is noteworthy for its ability to bind transition metal ions.
It is a styrene-divinylbenzene co-polymer containing iminodiacetic acid groups.
A conc ...
, which contains chelating ligands bound to a polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
, can be used in water softener
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extend ...
s and in chromatographic
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
separation techniques. In solvent extraction the formation of electrically-neutral complexes allows cations to be extracted into organic solvents. For example
Example may refer to:
* '' exempli gratia'' (e.g.), usually read out in English as "for example"
* .example
The name example is reserved by the Internet Engineering Task Force (IETF) as a domain name that may not be installed as a top-level ...
, in nuclear fuel reprocessing uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
(VI) and plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
(VI) are extracted into kerosene as the complexes O2(TBP)2(NO3)2(TBP = tri-''n''-butyl phosphate). In phase-transfer catalysis, a substance which is insoluble in an organic solvent can be made soluble by addition of a suitable ligand. For example, potassium permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution.
Potassium permanganate is widely used in the c ...
oxidations can be achieved by adding a catalytic quantity of a crown ether and a small amount of organic solvent to the aqueous reaction mixture, so that the oxidation reaction occurs in the organic phase.
In all these examples, the ligand is chosen on the basis of the stability constants of the complexes formed. For example, TBP is used in nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergo ...
reprocessing because (among other reasons) it forms a complex strong enough for solvent extraction to take place, but weak enough that the complex can be destroyed by nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
to recover the uranyl
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula . It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may ...
cation as nitrato complexes, such as O2(NO3)4sup>2− back in the aqueous phase.
Supramolecular complexes
Supramolecular complexes are held together by hydrogen bonding, hydrophobic forces, van der Waals forces, π-π interactions, and electrostatic effects, all of which can be described as noncovalent bonding. Applications includemolecular recognition
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, halogen ...
, host–guest chemistry In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest ch ...
and anion sensors
A sensor is a device that produces an output signal for the purpose of sensing a physical phenomenon.
In the broadest definition, a sensor is a device, module, machine, or subsystem that detects events or changes in its environment and sends ...
.
A typical application in molecular recognition involved the determination of formation constants for complexes formed between a tripodal substituted urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
molecule and various saccharide
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ma ...
s. The study was carried out using a non-aqueous solvent and NMR chemical shift measurements. The object was to examine the selectivity with respect to the saccharides.
An example of the use of supramolecular complexes in the development of chemosensors is provided by the use of transition-metal ensembles to sense for ATP.
Anion complexation can be achieved by encapsulating the anion in a suitable cage. Selectivity can be engineered by designing the shape of the cage. For example, dicarboxylate anions could be encapsulated in the ellipsoidal cavity in a large macrocyclic structure containing two metal ions.
Experimental methods
The method developed by Bjerrum is still the main method in use today, though the precision of the measurements has greatly increased. Most commonly, a solution containing the metal ion and the ligand in a medium of highionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such a ...
is first acidified to the point where the ligand is fully protonated. This solution is then titrated, often by means of a computer-controlled auto-titrator, with a solution of CO2-free base. The concentration, or activity, of the hydrogen ion is monitored by means of a glass electrode. The data set used for the calculation has three components: a statement defining the nature of the chemical species that will be present, called the model of the system, details concerning the concentrations of the reagents used in the titration, and finally the experimental measurements in the form of titre and pH (or emf) pairs.
Other ion-selective electrodes (ISE) may be used. For example, a fluoride electrode may be used with the determination of stability complexes of fluoro-complexes of a metal ion.
It is not always possible to use an ISE. If that is the case, the titration can be monitored by other types of measurement. Ultraviolet–visible spectroscopy
UV spectroscopy or UV–visible spectrophotometry (UV–Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relativ ...
, fluorescence spectroscopy
Fluorescence spectroscopy (also known as fluorimetry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electro ...
and NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fi ...
are the most commonly used alternatives. Current practice is to take absorbance or fluorescence measurements at a range of wavelengths and to fit these data simultaneously. Various NMR chemical shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure o ...
s can also be fitted together.
The chemical model will include values of the protonation constants of the ligand, which will have been determined in separate experiments, a value for log ''K''w and estimates of the unknown stability constants of the complexes formed. These estimates are necessary because the calculation uses a non-linear least-squares algorithm. The estimates are usually obtained by reference to a chemically similar system. The stability constant databases can be very useful in finding published stability constant values for related complexes.
In some simple cases the calculations can be done in a spreadsheet. Otherwise, the calculations are performed with the aid of a general-purpose computer programs. The most frequently used programs are:
* Potentiometric and/or spectrophotometric data: PSEQUAD
*Potentiometric dataHYPERQUAD
BEST
ReactLab pH PRO
* Spectrophotometric data
SQUAD, SPECFIT
ReactLab EQUILIBRIA
* NMR dat
In biochemistry, formation constants of adducts may be obtained from
Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) is a physical technique used to determine the thermodynamic parameters of interactions in solution. It is most often used to study the binding of small molecules (such as medicinal compounds) to larger macro ...
(ITC) measurements. This technique yields both the stability constant and the standard enthalpy change for the equilibrium. It is mostly limited, by availability of software, to complexes of 1:1 stoichiometry.
Critically evaluated data
The following references are for critical reviews of published stability constants for various classes of ligands. All these reviews are published byIUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
and the full text is available, free of charge, in pdf format.
*ethylenediamine
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately ...
(en)
*Nitrilotriacetic acid
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid that is used as a chelating agent, which forms coordination compounds with metal ions (chelates) such as Ca2+, Co2+, Cu2+, and Fe ...
(NTA)
*aminopolycarboxylic acid left, 120px, a metal complex with the EDTA anion
120px, Aspartic acid is an aminodicarboxylic acid and precursor to other ligands.
An aminopolycarboxylic acid (sometimes abbreviated APCA) is a chemical compound containing one or more nitrogen at ...
s (complexones)
*Alpha hydroxy acid
α-Hydroxy acids, or alpha hydroxy acids (AHAs), are a class of chemical compounds that consist of a carboxylic acid with a hydroxyl group substituent on the adjacent (alpha) carbon. Prominent examples are glycolic acid, lactic acid, mandelic ac ...
s and other hydroxycarboxylic acids
*crown ether
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . Impo ...
s
* phosphonic acids
*imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non ...
s and histamine
Histamine is an organic nitrogenous compound involved in local immune responses, as well as regulating physiological functions in the gut and acting as a neurotransmitter for the brain, spinal cord, and uterus. Since histamine was discover ...
s
*amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s with polar side-chains
*nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules with ...
*acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are tre ...
*general
*Chemical speciation of environmentally significant heavy metals with inorganic ligands. Part 1: The Hg2+–Cl−, OH−, , , and systems.
*Chemical speciation of environmentally significant metals with inorganic ligands Part 2: The Cu2+–OH−, Cl−, , , and aqueous systems
*Chemical speciation of environmentally significant metals with inorganic ligands Part 3: The Pb2+–OH−, Cl−, , , and systems
*Chemical speciation of environmentally significant metals with inorganic ligands. Part 4: The Cd2+–OH−, Cl−, , , and systems
Databases
*TheKi Database
The Ki Database (or Ki DB) is a public domain database of published binding affinities (''K''i) of drugs and chemical compounds for receptors, neurotransmitter transporters, ion channels, and enzymes. The resource is maintained by the University ...
is a public domain database of published binding affinities (Ki) of drugs and chemical compounds for receptors, neurotransmitter transporters, ion channels, and enzymes.
* BindingDB is a public domain database of measured binding affinities, focusing chiefly on the interactions of protein considered to be drug-targets with small, drug-like molecules
References
Further reading
External links
Stability constants website
Contains information on computer programs, applications, databases and hardware for experimental titrations. {{Chemical equilibria Equilibrium chemistry Coordination chemistry