Solubilization on:
[Wikipedia]
[Google]
[Amazon]
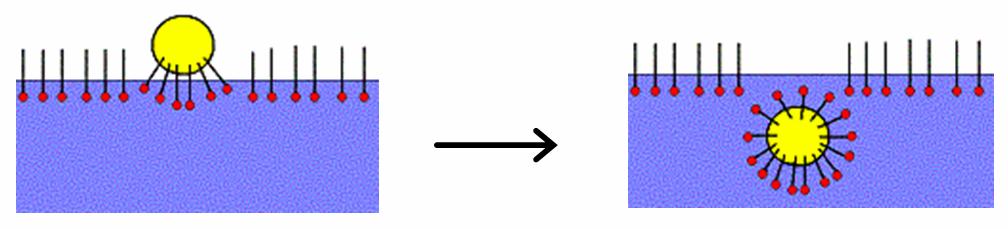 Micellar solubilization (solubilization) is the process of incorporating the solubilizate (the component that undergoes solublization) into or onto
Micellar solubilization (solubilization) is the process of incorporating the solubilizate (the component that undergoes solublization) into or onto
/ref> surface reaction, i.e., by transient adsorption of micelles at the water-oil interface, and bulk reaction, whereby the surfactant micelles capture dissolved oil molecules.
 Micellar solubilization (solubilization) is the process of incorporating the solubilizate (the component that undergoes solublization) into or onto
Micellar solubilization (solubilization) is the process of incorporating the solubilizate (the component that undergoes solublization) into or onto micelle
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated coll ...
s. Solublization may occur in a system consisting of a solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
, an association colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...
(a colloid that forms micelles), and at least one other solubilizate.
Usage of the term
Solubilization is distinct fromdissolution
Dissolution may refer to:
Arts and entertainment Books
* ''Dissolution'' (''Forgotten Realms'' novel), a 2002 fantasy novel by Richard Lee Byers
* ''Dissolution'' (Sansom novel), a 2003 historical novel by C. J. Sansom Music
* Dissolution, in mu ...
because the resulting fluid is a colloidal dispersion involving an association colloid. This suspension is distinct from a true solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Solutio ...
, and the amount of the solubilizate in the micellar system can be different (often higher) than the regular solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
of the solubilizate in the solvent.
In non-chemical literature and in everyday language, the term "solubilization" is sometimes used in a broader meaning as "to bring to a solution or (non- sedimenting) suspension" by any means, e.g., leaching by a reaction with an acid.
Application
Micellar solubilization is widely utilized, e.g. inlaundry
Laundry refers to the washing of clothing and other textiles, and, more broadly, their drying and ironing as well. Laundry has been part of history since humans began to wear clothes, so the methods by which different cultures have dealt with ...
washing
Washing is a method of cleaning, usually with water and soap or detergent. Washing and then rinsing both body and clothing is an essential part of good hygiene and health.
Often people use soaps and detergents to assist in the emulsification o ...
using detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are m ...
s, in the pharmaceutical industry, for formulations of poorly soluble drugs in solution form, and in cleanup of oil spill
An oil spill is the release of a liquid petroleum hydrocarbon into the environment, especially the marine ecosystem, due to human activity, and is a form of pollution. The term is usually given to marine oil spills, where oil is released into t ...
s using dispersant
A dispersant or a dispersing agent is a substance, typically a surfactant, that is added to a suspension of solid or liquid particles in a liquid (such as a colloid or emulsion) to improve the separation of the particles and to prevent their sett ...
s.
Mechanism
Literature distinguishes two major mechanisms of solubilization process of oil by surfactant micelles, affecting the kinetics of solubilization:P. D. Todorov, P. A. Kralchevsky, N. D. Denkov, G. Broze, and A. Mehreteab, "Kinetics of Solubilization of n-Decane and Benzene by Micellar Solutions of Sodium Dodecyl Sulfate". Journal of Colloid and Interface Science 245, 371–382 (2002),/ref> surface reaction, i.e., by transient adsorption of micelles at the water-oil interface, and bulk reaction, whereby the surfactant micelles capture dissolved oil molecules.
See also
*Hydrotrope A hydrotrope is a compound that solubilizes hydrophobic compounds in aqueous solutions by means other than micellar solubilization. Typically, hydrotropes consist of a hydrophilic part and a hydrophobic part (similar to surfactants), but the hydro ...
References
External links
{{Wiktionary, solubilization, solubilizate Solubilization of Homopolymers by Block Copolymer Micelles in Dilute Solutions, J. Phys. Chem., 1995, 99 (11), pp 3723–3731, Jose R. Quintana, Ramiro A. Salazar, Issa Katime Colloidal chemistry Solutions