Sandmeyer reaction on:
[Wikipedia]
[Google]
[Amazon]
The Sandmeyer reaction is a  The reaction was discovered in 1884 by Swiss chemist
The reaction was discovered in 1884 by Swiss chemist



 Some examples of the synthetic applications of the Sandmeyer reaction are provided below.
Some examples of the synthetic applications of the Sandmeyer reaction are provided below.
 One bromination protocol employs a Cu(I)/Cu(II) mixture with additional amounts of the bidentate
One bromination protocol employs a Cu(I)/Cu(II) mixture with additional amounts of the bidentate 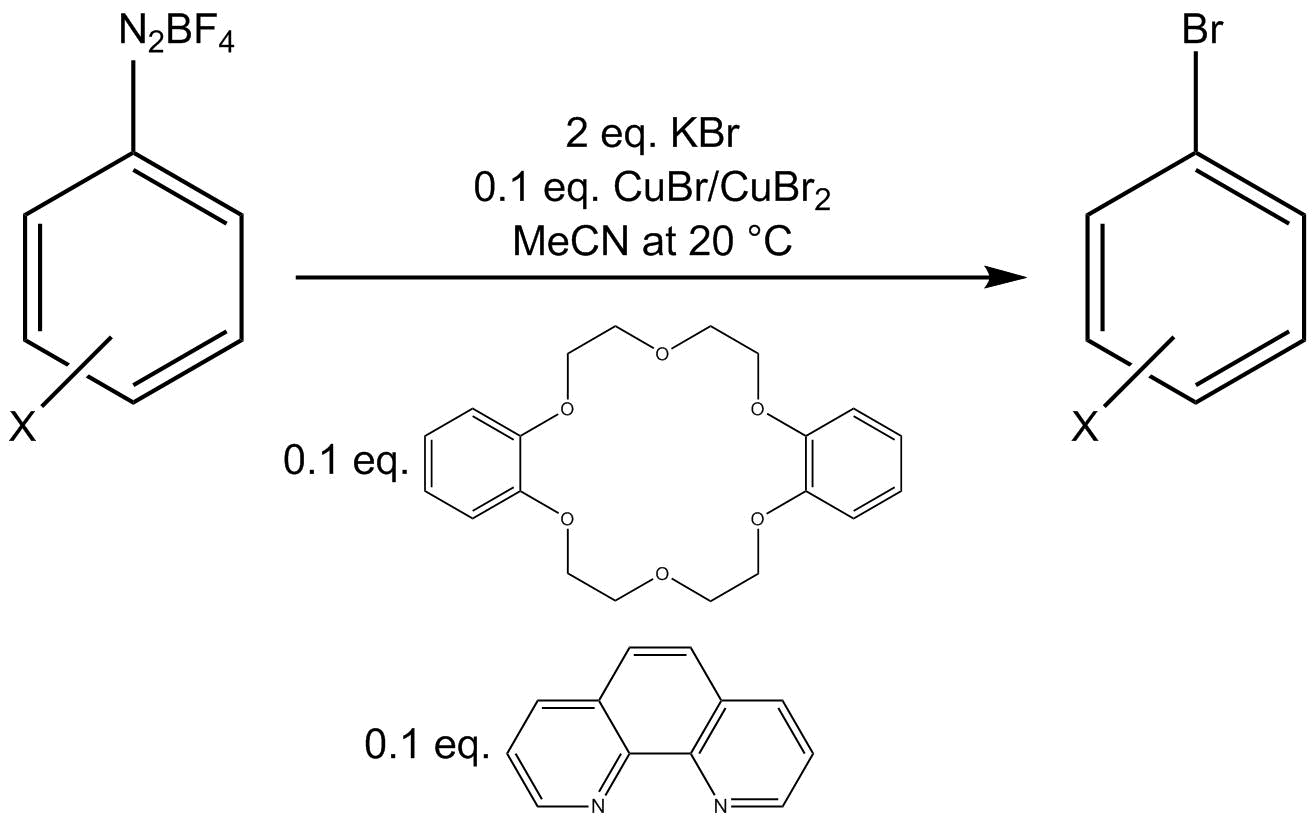 The
The
 The Sandmeyer reaction has also been employed in the synthesis of neoamphimedine, a compound that is suggested to target topoisomerase II as an anti-cancer drug.
The Sandmeyer reaction has also been employed in the synthesis of neoamphimedine, a compound that is suggested to target topoisomerase II as an anti-cancer drug.



chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking ...
used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts.
It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers ...
, cyanation In organic synthesis, cyanation is the attachment or substitution of a cyanide group on various substrates. Such transformations are high-value because they generate C-C bond. Furthermore nitriles are versatile functional groups.
Cyanation to fo ...
, trifluoromethylation Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound. Trifluoromethylated compounds are of some importance in pharmaceutical industry and agrochemicals. Several notab ...
, and hydroxylation
In chemistry, hydroxylation can refer to:
*(i) most commonly, hydroxylation describes a chemical process that introduces a hydroxyl group () into an organic compound.
*(ii) the ''degree of hydroxylation'' refers to the number of OH groups in a ...
.
: The reaction was discovered in 1884 by Swiss chemist
The reaction was discovered in 1884 by Swiss chemist Traugott Sandmeyer
Traugott Sandmeyer (15 September 1854 – 9 April 1922) was a Swiss chemist after whom the Sandmeyer reaction, which he discovered 1884, was named.
Life
Sandmeyer was born as the last of seven children and attended school in Aarau, studying ...
, when he attempted to synthesize phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
In ...
from benzenediazonium chloride and copper(I) acetylide
Copper(I) acetylide, or cuprous acetylide, is a chemical compound with the formula Cu2 C2. Although never characterized by X-ray crystallography, the material has been claimed at least since 1856. One form is claimed to be a monohydrate with for ...
. Instead, the main product he isolated was chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
Uses
Historical
The major use of chlorob ...
. In modern times, the Sandmeyer reaction refers to any method for substitution of an aromatic amino group via preparation of its diazonium salt followed by its displacement with a nucleophile in the presence of catalytic copper(I) salts. (Due to the low cost of copper salts, a stoichiometric amount is often employed for better reactivity even when catalysis is possible.) The most commonly employed Sandmeyer reactions are the chlorination, bromination, cyanation, and hydroxylation reactions using CuCl, CuBr, CuCN
CUCN or China-US Cable Network was a submarine communications cable, submarine telecommunications cable linking several countries in the Asia-Pacific region. It was retired from service in December 201
It has cable landing point, landing points in ...
, and Cu2O, respectively. More recently, trifluoromethylation of diazonium salts has been developed and is referred to as a 'Sandmeyer-type' reaction. Diazonium salts also react with boronates, iodide, thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
s, water, hypophosphorous acid
Hypophosphorous acid (HPA), or phosphinic acid, is a phosphorus oxyacid and a powerful reducing agent with molecular formula H3PO2. It is a colorless low-melting compound, which is soluble in water, dioxane
and alcohols. The formula for this ...
and others, and fluorination can be carried out using tetrafluoroborate anions (Balz–Schiemann reaction
The Balz–Schiemann reaction (also called the Schiemann reaction) is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate. This reaction is a traditional route to ...
). However, since these processes do not require a metal catalyst, they are not usually referred to as Sandmeyer reactions. In numerous variants that have been developed, other transition metal salts, including copper(II), iron(III) and cobalt(III) have also been employed. Due to its wide synthetic applicability, the Sandmeyer reaction, along with other transformations of diazonium compounds, is complementary to electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
.
Reaction conditions and mechanism
Thenitrous acid
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in solution, in the gas phase and in the form of nitrite () salts. Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagent ...
is typically prepared ''in situ'' from sodium nitrite
Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite ...
and acid. Following two protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
steps, one equivalent of water is lost to form the nitrosonium ion. The nitrosonium ion then acts as an electrophile in a reaction with an aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
(or heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
) amine, such as aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
, to form a diazonium salt, proceeding through a nitrosamine
In organic chemistry, nitrosamines (or more formally ''N''-Nitrosamines) are organic compounds with the chemical structure , where R is usually an alkyl group. They feature a nitroso group () bonded to a deprotonated amine. Most nitrosamines a ...
intermediate. Typical reaction conditions are as follows:
*''Chlorination:'' , CuCl, HCl HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a s ...
(36% aq.), 50–100 °C;
*''Bromination:'' , CuBr, HBr (48% aq.), 50–100 °C;
*''Cyanation:'' , CuCN
CUCN or China-US Cable Network was a submarine communications cable, submarine telecommunications cable linking several countries in the Asia-Pacific region. It was retired from service in December 201
It has cable landing point, landing points in ...
, KCN, H2O, benzene, 0 °C;
*''Hydroxylation:'' Cu2O, Cu(NO3)2, H2O, 25 °C.
The Sandmeyer reaction is an example of a radical-nucleophilic aromatic substitution (SRNAr). The radical mechanism of the Sandmeyer reaction is supported by the detection of biaryl byproducts. The substitution of the aromatic diazo group with a halogen or pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic ...
is initiated by a one-electron transfer mechanism catalyzed by copper(I) to form an aryl radical with loss of nitrogen gas. The substituted arene is possibly formed by direct transfer of Cl, Br, CN, or OH from a copper(II) species to the aryl radical to produce the substituted arene and regenerate the copper(I) catalyst. In an alternative proposal, a transient copper(III) intermediate, formed from coupling of the aryl radical with the copper(II) species, undergoes rapid reductive elimination to afford the product and regenerate copper(I). However, evidence for such an organocopper intermediate is weak and mostly circumstantial, and the exact pathway may depend on the substrate and reaction conditions. These possibilities are shown below.
Generation of the nitrosonium ion

Formation of the benzenediazonium ion
:Single electron transfer

Synthetic applications
Variations on the Sandmeyer reaction have been developed to fit multiple synthetic applications. These reactions typically proceed through the formation of an aryl diazonium salt followed by a reaction with a copper(I) salt to yield a substituted arene according to the scheme below.Halogenation
One of the most important uses of the Sandmeyer reaction is the formation of aryl halides. The solvent of choice for the synthesis of iodoarenes is diiodomethane, while for the synthesis of bromoarenes,bromoform
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, ...
is used. For the synthesis of chloroarenes In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhi ...
, chloroform
Chloroform, or trichloromethane, is an organic compound with formula C H Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various ...
is the solvent of choice. The synthesis of (+)- curcuphenol, a bioactive compound that displays antifungal and anticancer activity, employs the Sandmeyer reaction to substitute an amine group by a bromo group.
 One bromination protocol employs a Cu(I)/Cu(II) mixture with additional amounts of the bidentate
One bromination protocol employs a Cu(I)/Cu(II) mixture with additional amounts of the bidentate ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
phenanthroline
1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refer to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene.
Abbreviate ...
and phase-transfer catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of heterogeneous catalysis. Ionic reac ...
dibenzo-18-crown-6 to convert an aryl diazonium tetrafluoroborate salt to an aryl bromide.
: The
The Balz–Schiemann reaction
The Balz–Schiemann reaction (also called the Schiemann reaction) is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate. This reaction is a traditional route to ...
uses tetrafluoroborate and delivers the halide-substituted product, fluorobenzene
Fluorobenzene is the chemical compound with the formula C6H5F, often abbreviated PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds.
Preparation
PhF was first reported in 1886 by O. Wallach at the University of Bonn, who ...
, which is not obtained by the use of copper fluorides. This reaction displays motifs characteristic of the Sandmeyer reaction.
Cyanation
Another use of the Sandmeyer reaction is forcyanation In organic synthesis, cyanation is the attachment or substitution of a cyanide group on various substrates. Such transformations are high-value because they generate C-C bond. Furthermore nitriles are versatile functional groups.
Cyanation to fo ...
which allows for the formation of benzonitriles, an important class of organic compounds. A key intermediate in the synthesis of the antipsychotic drug Fluanxol is synthesized by a cyanation through the Sandmeyer reaction.
 The Sandmeyer reaction has also been employed in the synthesis of neoamphimedine, a compound that is suggested to target topoisomerase II as an anti-cancer drug.
The Sandmeyer reaction has also been employed in the synthesis of neoamphimedine, a compound that is suggested to target topoisomerase II as an anti-cancer drug.

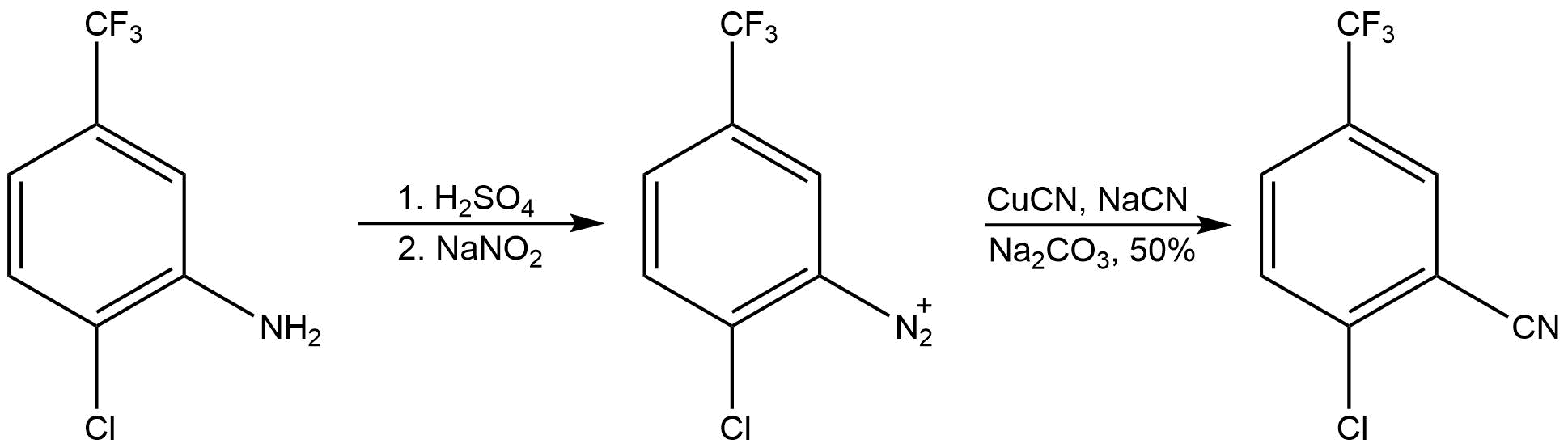
Trifluoromethylation
It has been demonstrated that Sandmeyer-type reactions can be used to generate aryl compounds functionalized by trifluoromethyl substituent groups. This process oftrifluoromethylation Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound. Trifluoromethylated compounds are of some importance in pharmaceutical industry and agrochemicals. Several notab ...
provides unique chemical properties with a wide variety of practical applications. Particularly, pharmaceuticals with CF3 groups have enhanced metabolic stability, lipophilicity
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lip ...
, and bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. Ho ...
. Sandmeyer-type trifluoromethylation reactions feature mild reaction conditions and greater functional group tolerance relative to earlier methods of trifluoromethylation. An example of a Sandmeyer-type trifluoromethylation reaction is presented below.

Hydroxylation
The Sandmeyer reaction can also be used to convert aryl amines tophenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it r ...
s proceeding through the formation of an aryl diazonium salt as shown below. In the presence of copper catalyst, this reaction takes place readily at room temperature. The procedure reported by Cohen and coworkers calls for copper(I) oxide together with an excess of copper(II) nitrate in neutral water. This is in contrast to the classical procedure (known by the German name '' Verkochung''), which calls for boiling the diazonium salt in aqueous acid, a process that is believed to involve the aryl cation instead of radical and is known to generate other nucleophilic addition side products in addition to the desired hydroxylation product.

Triazene
Treatment with a second equivalent ofaniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
would give a triazene. Compare, for example, diminazene (Berenil) and isometamidium chloride.
References
External links
* http://www.name-reaction.com/sandmeyer-reaction {{DEFAULTSORT:Sandmeyer Reaction Substitution reactions Name reactions