Resonance-stabilized on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 For instance, in NO2â,
For instance, in NO2â,
A<->B is used to indicate that A and B are contributing forms of a single chemical species (as opposed to an equilibrium arrow, e.g., A <=> B ; see
 In
In 
 For hypervalent molecules, the rationalization described above can be applied to generate contributing structures to explain the bonding in such molecules. Shown below are the contributing structures of a 3c-4e bond in xenon difluoride.
:
For hypervalent molecules, the rationalization described above can be applied to generate contributing structures to explain the bonding in such molecules. Shown below are the contributing structures of a 3c-4e bond in xenon difluoride.
: mathsf/chem>
 The diborane molecule is described by contributing structures, each with electron-deficiency on different atoms. This reduces the electron-deficiency on each atom and stabilizes the molecule. Below are the contributing structures of an individual 3c-2e bond in diborane.
:
The diborane molecule is described by contributing structures, each with electron-deficiency on different atoms. This reduces the electron-deficiency on each atom and stabilizes the molecule. Below are the contributing structures of an individual 3c-2e bond in diborane.
:
 Hydrogenation of one mole of double bonds delivers 119.7 kJ (28.6 kcal), as can be deduced from the last step, the hydrogenation of cyclohexene. In benzene, however, 23.4 kJ (5.6 kcal) are needed to hydrogenate one mole of double bonds. The difference, being 143.1 kJ (34.2 kcal), is the empirical resonance energy of benzene. Because 1,3-cyclohexadiene also has a small delocalization energy (7.6 kJ or 1.8 kcal/mol) the net resonance energy, relative to the localized cyclohexatriene, is a bit higher: 151 kJ or 36 kcal/mol.
This measured resonance energy is also the difference between the hydrogenation energy of three 'non-resonance' double bonds and the measured hydrogenation energy:
:(3 Ã 119.7) â 208.4 = 150.7 kJ/mol (36 kcal).
Hydrogenation of one mole of double bonds delivers 119.7 kJ (28.6 kcal), as can be deduced from the last step, the hydrogenation of cyclohexene. In benzene, however, 23.4 kJ (5.6 kcal) are needed to hydrogenate one mole of double bonds. The difference, being 143.1 kJ (34.2 kcal), is the empirical resonance energy of benzene. Because 1,3-cyclohexadiene also has a small delocalization energy (7.6 kJ or 1.8 kcal/mol) the net resonance energy, relative to the localized cyclohexatriene, is a bit higher: 151 kJ or 36 kcal/mol.
This measured resonance energy is also the difference between the hydrogenation energy of three 'non-resonance' double bonds and the measured hydrogenation energy:
:(3 Ã 119.7) â 208.4 = 150.7 kJ/mol (36 kcal).
 Resonance has a deeper significance in the mathematical formalism of
Resonance has a deeper significance in the mathematical formalism of
 In molecular orbital theory, the main alternative to
In molecular orbital theory, the main alternative to
NBO5
, or finally from empirical calculations based on the HÞckel method. A HÞckel method-based software for teaching resonance is available on th
HuLiS
Web site.
 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, resonance, also called mesomerism, is a way of describing bonding in certain molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
or polyatomic ions
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may not ...
by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure.
Overview
Under the framework ofvalence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charge
In chemistry, a formal charge (F.C. or q), in the covalent view of chemical bonding, is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electroneg ...
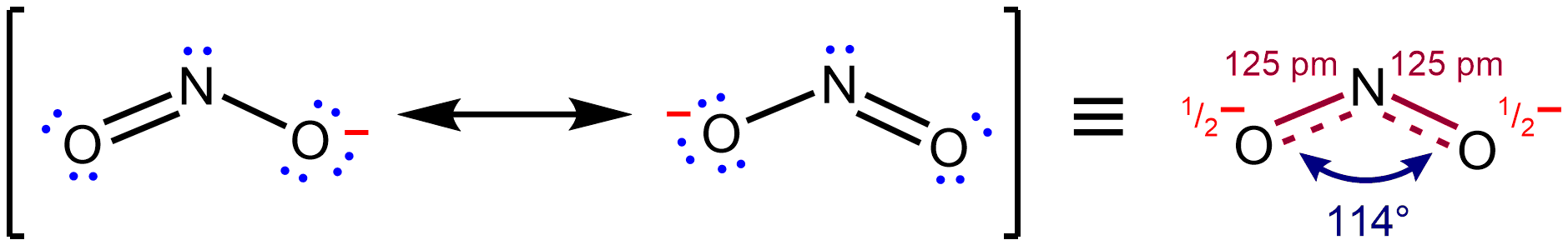
s, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. However, in some cases, more than one Lewis structure could be drawn, and experimental properties are inconsistent with any one structure. In order to address this type of situation, several contributing structures are considered together as an average, and the molecule is said to be represented by a resonance hybrid in which several Lewis structures are used collectively to describe its true structure.  For instance, in NO2â,
For instance, in NO2â, nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
anion, the two NâO bond lengths are equal, even though no single Lewis structure has two NâO bonds with the same formal bond order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diat ...
. However, its measured structure is consistent with a description as a resonance hybrid of the two major contributing structures shown above: it has two ''equal'' NâO bonds of 125 pm, intermediate in length between a typical NâO single bond (145 pm in hydroxylamine, H2NâOH) and NâO double bond (115 pm in nitronium ion
The nitronium ion, , is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion . It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule , or the protonation of nitric aci ...
, =N=Osup>+). According to the contributing structures, each NâO bond is an average of a formal single and formal double bond, leading to a true bond order of 1.5. By virtue of this averaging, the Lewis description of the bonding in NO2â is reconciled with the experimental fact that the anion has equivalent NâO bonds.
The resonance hybrid represents the actual molecule as the "average" of the contributing structures, with bond lengths and partial charges taking on intermediate values compared to those expected for the individual Lewis structures of the contributors, were they to exist as "real" chemical entities. The contributing structures differ only in the ''formal'' apportionment of electrons to the atoms, and not in the actual physically and chemically significant electron or spin density. While contributing structures may differ in formal bond orders and in formal charge
In chemistry, a formal charge (F.C. or q), in the covalent view of chemical bonding, is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electroneg ...
assignments, all contributing structures must have the same number of valence electrons and the same spin multiplicity
Multiplicity may refer to: In science and the humanities
* Multiplicity (mathematics), the number of times an element is repeated in a multiset
* Multiplicity (philosophy), a philosophical concept
* Multiplicity (psychology), having or using multi ...
.
Because electron delocalization lowers the potential energy of a system, any species represented by a resonance hybrid is more stable than any of the (hypothetical) contributing structures. Electron delocalization stabilizes a molecule because the electrons are more evenly spread out over the molecule, decreasing electron-electron repulsion. The difference in potential energy between the actual species and the (computed) energy of the contributing structure with the lowest potential energy is called the ''resonance energy'' or delocalization energy. The magnitude of the resonance energy depends on assumptions made about the hypothetical "non-stabilized" species and the computational methods used and does not represent a measurable physical quantity, although comparisons of resonance energies computed under similar assumptions and conditions may be chemically meaningful.
Molecules with an extended Ï system such as linear polyenes and polyaromatic compounds are well described by resonance hybrids as well as by delocalised orbitals in molecular orbital theory.
Resonance vs isomerism
Resonance is to be distinguished fromisomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae â that is, same number of atoms of each element â but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
ism. Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae â that is, same number of atoms of each element â but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
s are molecules with the same chemical formula but are distinct chemical species with different arrangements of atomic nuclei in space. Resonance contributors of a molecule, on the other hand, can only differ in the way electrons are formally assigned to atoms in the Lewis structure ''depictions'' of the molecule. Specifically, when a molecular structure is said to be represented by a resonance hybrid, it does ''not'' mean that electrons of the molecule are "resonating" or shifting back and forth between several sets of positions, each one represented by a Lewis structure. Rather, it means that the set of contributing structures ''represents an intermediate structure'' (a weighted average of the contributors), with a single, well-defined geometry and distribution of electrons. It is incorrect to regard resonance hybrids as rapidly interconverting isomers, even though the term "resonance" might evoke such an image. (As described below
Below may refer to:
*Earth
*Ground (disambiguation)
*Soil
*Floor
*Bottom (disambiguation)
Bottom may refer to:
Anatomy and sex
* Bottom (BDSM), the partner in a BDSM who takes the passive, receiving, or obedient role, to that of the top or ...
, the term "resonance" originated as a classical physics analogy for a quantum mechanical phenomenon, so it should not be construed too literally.) Symbolically, the double headed arrow below
Below may refer to:
*Earth
*Ground (disambiguation)
*Soil
*Floor
*Bottom (disambiguation)
Bottom may refer to:
Anatomy and sex
* Bottom (BDSM), the partner in a BDSM who takes the passive, receiving, or obedient role, to that of the top or ...
for details on usage).
A non-chemical analogy is illustrative: one can describe the characteristics of a real animal, the narwhal
The narwhal, also known as a narwhale (''Monodon monoceros''), is a medium-sized toothed whale that possesses a large "tusk" from a protruding canine tooth. It lives year-round in the Arctic waters around Greenland, Canada and Russia. It is o ...
, in terms of the characteristics of two mythical creatures: the unicorn, a creature with a single horn on its head, and the leviathan, a large, whale-like creature. The narwhal is not a creature that goes back and forth between being a unicorn and being a leviathan, nor do the unicorn and leviathan have any physical existence outside the collective human imagination. Nevertheless, describing the narwhal in terms of these imaginary creatures provides a reasonably good description of its physical characteristics.
Due to confusion with the physical meaning of the word resonance, as no entities actually physically "resonate", it has been suggested that the term resonance be abandoned in favor of ''delocalization'' and resonance energy abandoned in favor of ''delocalization energy''. A resonance structure becomes a ''contributing structure'' and the resonance hybrid becomes the ''hybrid structure''. The double headed arrows would be replaced by commas to illustrate a set of structures, as arrows of any type may suggest to beginning students that a chemical change is taking place.
Representation in diagrams
In diagrams, contributing structures are typically separated by double-headed arrows (â). The arrow should not be confused with the right and left pointing ''equilibrium arrow'' (). All structures together may be enclosed in large square brackets, to indicate they picture one single molecule or ion, not different species in a chemical equilibrium. Alternatively to the use of contributing structures in diagrams, a hybrid structure can be used. In a hybrid structure,pi bonds
In chemistry, pi bonds (Ï bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
that are involved in resonance are usually pictured as curves
or dashed lines, indicating that these are partial rather than normal complete pi bonds. In benzene and other aromatic rings, the delocalized pi-electrons are sometimes pictured as a solid circle.
History
The concept first appeared in 1899 inJohannes Thiele Johannes Thiele may refer to:
*Johannes Thiele (zoologist)
*Johannes Thiele (chemist)
{{hndis, Thiele, Johannes ...
's "Partial Valence Hypothesis" to explain the unusual stability of benzene which would not be expected from August KekulÃĐ's structure proposed in 1865 with alternating single and double bonds. Benzene undergoes substitution reactions, rather than addition reactions as typical for alkenes. He proposed that the carbon-carbon bond in benzene is intermediate of a single and double bond.
The resonance proposal also helped explain the number of isomers of benzene derivatives. For example, KekulÃĐ's structure would predict ''four'' dibromobenzene isomers, including two ortho isomers with the brominated carbon atoms joined by either a single or a double bond. In reality there are only three dibromobenzene isomers and only one is ortho, in agreement with the idea that there is only one type of carbon-carbon bond, intermediate between a single and a double bond.
The mechanism of resonance was introduced into quantum mechanics by Werner Heisenberg in 1926 in a discussion of the quantum states of the helium atom. He compared the structure of the helium atom with the classical system of resonating coupled harmonic oscillator
In classical mechanics, a harmonic oscillator is a system that, when displaced from its Mechanical equilibrium, equilibrium position, experiences a restoring force ''F'' Proportionality (mathematics), proportional to the displacement ''x'':
\v ...
s. In the classical system, the coupling produces two modes, one of which is lower in frequency than either of the uncoupled vibrations; quantum mechanically, this lower frequency is interpreted as a lower energy. Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific top ...
used this mechanism to explain the partial valence of molecules in 1928, and developed it further in a series of papers in 1931-1933. The alternative term ''mesomerism'' popular in German and French publications with the same meaning was introduced by C. K. Ingold
Sir Christopher Kelk Ingold (28 October 1893 â 8 December 1970) was a British chemist based in Leeds and London. His groundbreaking work in the 1920s and 1930s on reaction mechanisms and the electronic structure of organic compounds was resp ...
in 1938, but did not catch on in the English literature. The current concept of mesomeric effect has taken on a related but different meaning. The double headed arrow was introduced by the German chemist Fritz Arndt
Fritz Georg Arndt (6 July 1885 â 8 December 1969) was a German chemist recognised for his contributions to synthetic methodology, who together with Bernd Eistert discovered the Arndt-Eistert synthesis.
Life
Fritz Arndt was born on 6 July ...
who preferred the German phrase ''zwischenstufe'' or ''intermediate stage''.
In the Soviet Union, resonance theory â especially as developed by Pauling â was attacked in the early 1950s as being contrary to the Marxist principles of dialectical materialism
Dialectical materialism is a philosophy of science, history, and nature developed in Europe and based on the writings of Karl Marx and Friedrich Engels. Marxist dialectics, as a materialist philosophy, emphasizes the importance of real-world con ...
, and in June 1951 the Soviet Academy of Sciences under the leadership of Alexander Nesmeyanov convened a conference on the chemical structure of organic compounds, attended by 400 physicists, chemists, and philosophers, where "the pseudo-scientific essence of the theory of resonance was exposed and unmasked".
Major and minor contributors
One contributing structure may resemble the actual molecule more than another (in the sense of energy and stability). Structures with a low value of potential energy are more stable than those with high values and resemble the actual structure more. The most stable contributing structures are called ''major contributors''. Energetically unfavourable and therefore less favorable structures are ''minor contributors''. With rules listed in rough order of diminishing importance, major contributors are generally structures that #obey as much as possible the octet rule (8 valence electrons around each atom rather than having deficiencies or surplus, or 2 electrons forPeriod 1 element
A period 1 element is one of the chemical elements in the first row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate periodic (recurring) trends in the chemical behaviour of t ...
s);
#have a maximum number of covalent bonds;
#carry a minimum of formally charged atoms, with the separation for unlike and like charges minimized and maximized, respectively;
#place negative charge, if any, on the most electronegative atoms and positive charge, if any, on the most electropositive;
#do not deviate substantially from idealized bond lengths and angles (e.g., the relative unimportance of Dewar-type resonance contributors for benzene);
#maintain aromatic substructures locally while avoiding anti-aromatic ones (''see'' Clar sextet ''and'' biphenylene).
A maximum of eight valence electrons is strict for the Period 2 element
A period 2 element is one of the chemical elements in the second row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of th ...
s Be, B, C, N, O, and F, as is a maximum of two for H and He and effectively for Li as well. The issue of expansion of the valence shell of third period and heavier main group elements is controversial. A Lewis structure in which a central atom has a valence electron count greater than eight traditionally implies the participation of d orbitals in bonding. However, the consensus opinion is that while they may make a marginal contribution, the participation of d orbitals is unimportant, and the bonding of so-called hypervalent molecules are, for the most part, better explained by charge-separated contributing forms that depict three-center four-electron bonding. Nevertheless, by tradition, expanded octet structures are still commonly drawn for functional groups like sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s, sulfones, and phosphorus ylides, for example. Regarded as a formalism that does not necessarily reflect the true electronic structure, such depictions are preferred by the IUPAC over structures featuring partial bonds, charge separation, or dative bonds.
Equivalent contributors contribute equally to the actual structure, while the importance of nonequivalent contributors is determined by the extent to which they conform to the properties listed above. A larger number of significant contributing structures and a more voluminous space available for delocalized electrons lead to stabilization (lowering of the energy) of the molecule.
Examples
Aromatic molecules
In benzene the two cyclohexatriene ''KekulÃĐ'' structures, first proposed by KekulÃĐ, are taken together as contributing structures to represent the total structure. In the hybrid structure on the right, the dashed hexagon replaces three double bonds, and represents six electrons in a set of three molecular orbitals of Ï symmetry, with a nodal plane in the plane of the molecule. :furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
a lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of the oxygen atom interacts with the Ï orbitals of the carbon atoms. The curved arrow Arrow pushing or electron pushing is a technique used to describe the progression of organic chemistry reaction mechanisms. It was first developed by Sir Robert Robinson. In using arrow pushing, "curved arrows" or "curly arrows" are drawn on the ...
s depict the permutation of delocalized Ï electrons, which results in different contributors.
:Electron-rich molecules
The ozone molecule is represented by two contributing structures. In reality the two terminal oxygen atoms are equivalent and the hybrid structure is drawn on the right with a charge of â on both oxygen atoms and partial double bonds with a full and dashed line andbond order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diat ...
.
:Electron-deficient molecules
Theallyl cation
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . ...
has two contributing structures with a positive charge on the terminal carbon atoms. In the hybrid structure their charge is +. The full positive charge can also be depicted as delocalized among three carbon atoms.
:Reactive intermediates
Often, reactive intermediates such as carbocations andfree radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
have more delocalized structure than their parent reactants, giving rise to unexpected products. The classical example is allylic rearrangement An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution.
In reaction conditions that favor a SN1 reactio ...
. When 1 mole of HCl adds to 1 mole of 1,3-butadiene, in addition to the ordinarily expected product 3-chloro-1-butene, we also find 1-chloro-2-butene. Isotope labelling experiments have shown that what happens here is that the additional double bond shifts from 1,2 position to 2,3 position in some of the product. This and other evidence (such as NMR in superacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of â12. According to the modern definition, a superacid ...
solutions) shows that the intermediate carbocation must have a highly delocalized structure, different from its mostly classical (delocalization exists but is small) parent molecule. This cation (an allylic cation) can be represented using resonance, as shown above.
This observation of greater delocalization in less stable molecules is quite general. The excited states of conjugated dienes are stabilised more by conjugation than their ground states, causing them to become organic dyes.
A well-studied example of delocalization that does not involve Ï electrons ( hyperconjugation) can be observed in the non-classical 2-Norbornyl cation
In organic chemistry, the term 2-norbornyl cation (or 2-bicyclo .2.1eptyl cation) describes one of the three carbocations formed from derivatives of norbornane. Though 1-norbornyl and 7-norbornyl cations have been studied, the most extensive studie ...
. Another example is methanium (). These can be viewed as containing three-center two-electron bonds and are represented either by contributing structures involving rearrangement of Ï electrons or by a special notation, a Y that has the three nuclei at its three points.
Delocalized electrons are important for several reasons; a major one is that an expected chemical reaction may not occur because the electrons delocalize to a more stable configuration, resulting in a reaction that happens at a different location. An example is the FriedelâCrafts alkylation of benzene with 1-chloro-2-methylpropane; the carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
rearranges to a ''tert''- butyl group stabilized by hyperconjugation, a particular form of delocalization. Delocalization leads to lengthening of wavelength of electron therefore decreases the energy.
Benzene
Bond lengths
Comparing the two contributing structures of benzene, all single and double bonds are interchanged. Bond lengths can be measured, for example usingX-ray diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
. The average length of a CâC single bond is 154 pm; that of a C=C double bond is 133 pm. In localized cyclohexatriene, the carbonâcarbon bonds should be alternating 154 and 133 pm. Instead, all carbonâcarbon bonds in benzene are found to be about 139 pm, a bond length intermediate between single and double bond. This mixed single and double bond (or triple bond) character is typical for all molecules in which bonds have a different bond order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diat ...
in different contributing structures. Bond lengths can be compared using bond orders. For example, in cyclohexane the bond order is 1 while that in benzene is 1 + (3 ÷ 6) = . Consequently, benzene has more double bond character and hence has a shorter bond length than cyclohexane.
Resonance energy
Resonance (or delocalization) energy is the amount of energy needed to convert the true delocalized structure into that of the most stable contributing structure. The ''empirical resonance energy'' can be estimated by comparing theenthalpy change
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
of hydrogenation of the real substance with that estimated for the contributing structure.
The complete hydrogenation of benzene to cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
via 1,3-cyclohexadiene
Cyclohexa-1,3-diene is an organic compound with the formula (C2H4)(CH)4. It is a colorless, flammable liquid. Its refractive index is 1.475 (20 °C, D). A naturally occurring derivative of 1,3-cyclohexadiene is terpinene, a component of pine oi ...
and cyclohexene is exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
; 1 mole of benzene delivers 208.4 kJ (49.8 kcal).
Quantum mechanical description in valence bond (VB) theory
valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
(VB). Quantum mechanics requires that the wavefunction of a molecule obey its observed symmetry. If a single contributing structure does not achieve this, resonance is invoked.
For example, in benzene, valence bond theory begins with the two KekulÃĐ structures which do not individually possess the sixfold symmetry of the real molecule. The theory constructs the actual wave function as a linear superposition of the wave functions representing the two structures. As both KekulÃĐ structures have equal energy, they are equal contributors to the overall structure â the superposition is an equally weighted average, or a 1:1 linear combination of the two in the case of benzene. The symmetric combination gives the ground state, while the antisymmetric combination gives the first excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
, as shown.
In general, the superposition is written with undetermined coefficients, which are then variationally optimized to find the lowest possible energy for the given set of basis wave functions. When more contributing structures are included, the molecular wave function becomes more accurate and more excited states can be derived from different combinations of the contributing structures.
Comparison with molecular orbital (MO) theory
 In molecular orbital theory, the main alternative to
In molecular orbital theory, the main alternative to valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
, the molecular orbitals (MOs) are approximated as sums of all the atomic orbitals (AOs) on all the atoms; there are as many MOs as AOs. Each AO''i'' has a ''weighting'' coefficient ''ci'' that indicates the AO's contribution to a particular MO. For example, in benzene, the MO model gives us 6 Ï MOs which are combinations of the 2p''z'' AOs on each of the 6 C atoms. Thus, each Ï MO is delocalized over the whole benzene molecule and any electron ''occupying'' an MO will be delocalized over the whole molecule. This MO interpretation has inspired the picture of the benzene ring as a hexagon with a circle inside. When describing benzene, the VB concept of localized Ï bonds and the MO concept of delocalized Ï orbitals are frequently combined in elementary chemistry courses.
The contributing structures in the VB model are particularly useful in predicting the effect of substituents on Ï systems such as benzene. They lead to the models of contributing structures for an electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
and electron-releasing group on benzene. The utility of MO theory is that a quantitative indication of the charge from the Ï system on an atom can be obtained from the squares of the ''weighting'' coefficient ''ci'' on atom C''i''. Charge ''qi'' â ''c''. The reason for squaring the coefficient is that if an electron is described by an AO, then the square of the AO gives the electron density. The AOs are adjusted ( normalized) so that AO2 = 1, and ''qi'' â (''ci''AO''i'')2 â ''c''. In benzene, ''qi'' = 1 on each C atom. With an electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
''qi'' < 1 on the ''ortho'' and ''para'' C atoms and ''qi'' > 1 for an electron-releasing group.
Coefficients
Weighting of the contributing structures in terms of their contribution to the overall structure can be calculated in multiple ways, using ''"Ab initio"'' methods derived from Valence Bond theory, or else from the Natural Bond Orbitals (NBO) approaches of WeinholNBO5
, or finally from empirical calculations based on the HÞckel method. A HÞckel method-based software for teaching resonance is available on th
HuLiS
Web site.
Charge delocalization
In the case of ions it is common to speak about delocalized charge (charge delocalization). An example of delocalized charge in ions can be found in the carboxylate group, wherein the negative charge is centered equally on the two oxygen atoms. Charge delocalization in anions is an important factor determining their reactivity (generally: the higher the extent of delocalization the lower the reactivity) and, specifically, the acid strength of their conjugate acids. As a general rule, the better delocalized is the charge in an anion the stronger is its conjugate acid. For example, the negative charge in perchlorate anion () is evenly distributed among the symmetrically oriented oxygen atoms (and a part of it is also kept by the central chlorine atom). This excellent charge delocalization combined with the high number of oxygen atoms (four) and high electronegativity of the central chlorine atom leads to perchloric acid being one of the strongest known acids with a p''K''a value of â10. The extent of charge delocalization in an anion can be quantitatively expressed via the WAPS (weighted average positive sigma) parameter parameter and an analogous WANS (weighted average negative sigma) parameter is used for cations. WAPS and WANS values are given in e/ Ã 4. Larger values indicate more localized charge in the corresponding ion.See also
*Conjugated system
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as ...
* Delocalization
*HÞckel molecular orbital theory HÞckel or Huckel may refer to:
* Erich HÞckel (1896-1980), German physicist and chemist
** DebyeâHÞckel equation (named after Peter Debye and Erich HÞckel), in chemistry, a method of calculating activity coefficients
** HÞckel method (name ...
* Hyperconjugation
* Tautomerism
* Avoided crossing
External links
*References
{{DEFAULTSORT:Resonance (Chemistry) Chemical bonding Physical chemistry Electronic structure methods