Radon-222 on:
[Wikipedia]
[Google]
[Amazon]
Radon-222 (222Rn, Rn-222, historically radium emanation or radon) is the most stable
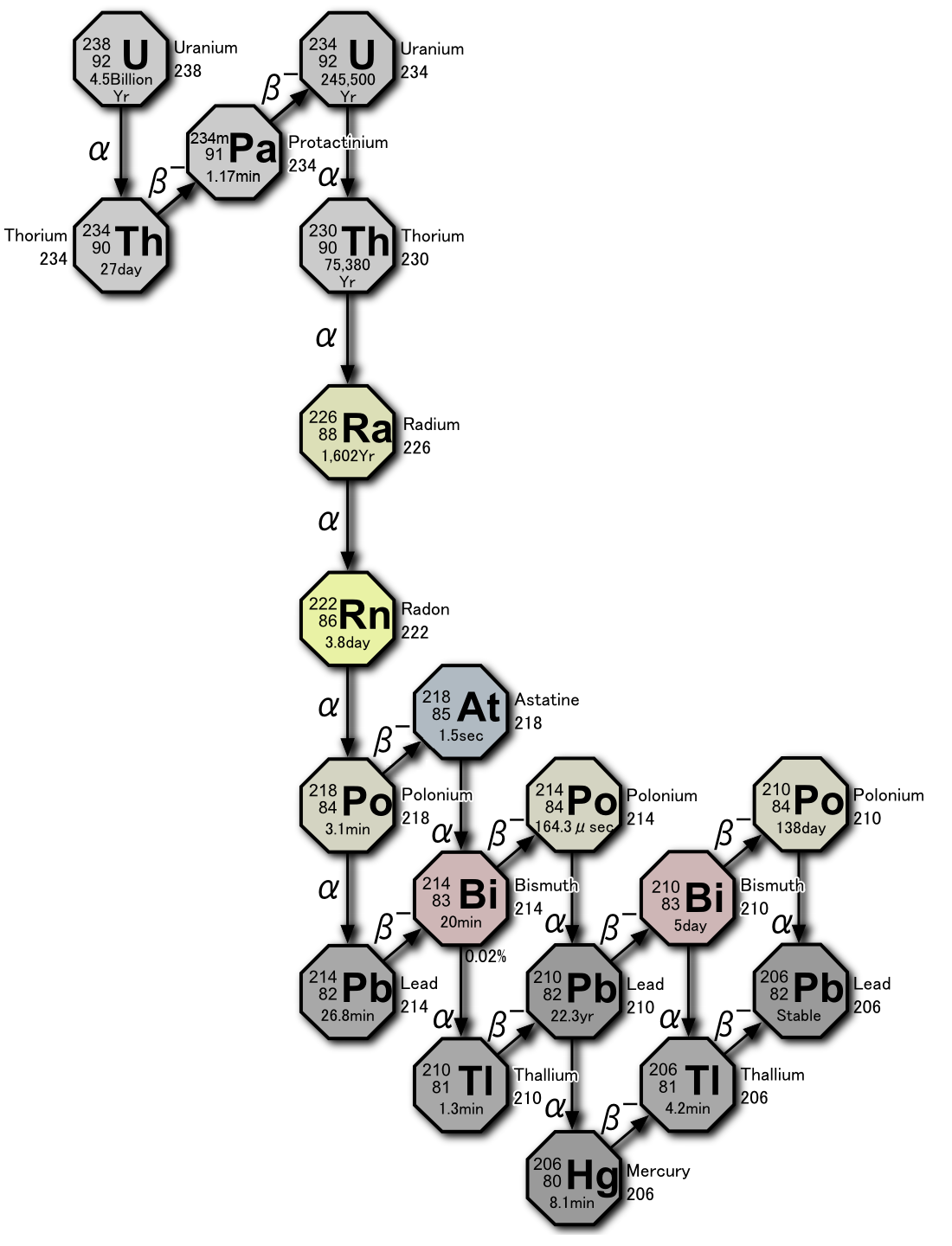 Radon-222 is generated in the uranium series from the
Radon-222 is generated in the uranium series from the
isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
of radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains th ...
, with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of approximately 3.8 days. It is transient in the decay chain
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay dire ...
of primordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial sea (a.k.a. primordial ocean, ooze or soup). See Abiogenesis
* Primordial nuclide, nuclides, a few radioactive, that formed before t ...
uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However ...
and is the immediate decay product of radium-226. Radon-222 was first observed in 1899, and was identified as an isotope of a new element several years later. In 1957, the name ''radon'', formerly the name of only radon-222, became the name of the element. Owing to its gaseous nature and high radioactivity, radon-222 is one of the leading causes of lung cancer
Lung cancer, also known as lung carcinoma (since about 98–99% of all lung cancers are carcinomas), is a malignant lung tumor characterized by uncontrolled cell growth in tissues of the lung. Lung carcinomas derive from transformed, mali ...
.
History
Following the 1898 discovery ofradium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rat ...
through chemical analysis of radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
ore, Marie and Pierre Curie
Pierre Curie ( , ; 15 May 1859 – 19 April 1906) was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity, and radioactivity. In 1903, he received the Nobel Prize in Physics with his wife, Marie Curie, and Henri Becq ...
observed a new radioactive substance emanating from radium in 1899 that was strongly radioactive for several days. Around the same time, Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson, (30 August 1871 – 19 October 1937) was a New Zealand physicist who came to be known as the father of nuclear physics.
''Encyclopædia Britannica'' considers him to be the greatest ...
and Robert B. Owens observed a similar (though shorter-lived) emission from thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
compounds. German physicist Friedrich Ernst Dorn extensively studied these emanations in the early 1900s and attributed them to a new gaseous element, radon. In particular, he studied the product in the uranium series
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay direct ...
, radon-222, which he called ''radium emanation''.
In the early 20th century, the element radon was known by several different names. Chemist William Ramsay
Sir William Ramsay (; 2 October 1852 – 23 July 1916) was a Scottish chemist who discovered the noble gases and received the Nobel Prize in Chemistry in 1904 "in recognition of his services in the discovery of the inert gaseous element ...
, who extensively studied the element's chemical properties, suggested the name ''niton'', and Rutherford originally suggested ''emanation''. At that time, ''radon'' only referred to the isotope 222Rn, whereas the names ''actinon'' and ''thoron'' denoted 219Rn and 220Rn, respectively. In 1957, the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC) promoted the name ''radon'' to refer to the element rather than just 222Rn; this was done under a new rule concerning isotope naming conventions. This decision was controversial because it was believed to give undue credit to Dorn's identification of radon-222 over Rutherford's identification of radon-220, and the historical use of the name radon created confusion as to whether the element or the isotope 222Rn was being discussed.
Decay properties
alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
of radium-226, which has a half-life of 1600 years. Radon-222 itself alpha decays to polonium-218 with a half-life of approximately 3.82 days, making it the most stable isotope of radon. Its final decay product is stable lead-206
Lead (82Pb) has four stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay chains: the urani ...
.
In theory, 222Rn is capable of double beta decay
In nuclear physics, double beta decay is a type of radioactive decay in which two neutrons are simultaneously transformed into two protons, or vice versa, inside an atomic nucleus. As in single beta decay, this process allows the atom to move clos ...
to 222Ra, and depending on the mass measurement, single beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
to 222Fr may also be allowed. These decay modes have been searched for, yielding lower partial half-life limits of 8 years for both transitions. If the beta decay of 222Rn is possible, it is predicted to have a very low decay energy (24 ± 21 keV) and thus a half-life on the order of 105 years, also resulting in a very low branching probability relative to alpha decay.
Occurrence and hazards
All radon isotopes are hazardous owing to their radioactivity, gaseous nature, chemical inertness, and radioactivity of their decay products (progeny). Radon-222 is especially dangerous because its longer half-life allows it to permeate soil and rocks, where it is produced in trace quantities from decays of uranium-238, and concentrate in buildings and uranium mines. This contrasts with the other natural isotopes that decay far more quickly (half-lives less than 1 minute) and thus do not contribute significantly to radiation exposure. At higher concentrations, gaseous 222Rn may be inhaled and decay before exhalation, which leads to a buildup of its daughters 218Po and 214Po in the lungs, whose high-energy alpha andgamma
Gamma (uppercase , lowercase ; ''gámma'') is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter r ...
radiation damages cells. Extended periods of exposure to 222Rn and its progeny ultimately induce lung cancer. Alternatively, radon may enter the body through contaminated drinking water or through the decay of ingested radium – making radon diffusion one of the greatest dangers of radium. Thus, 222Rn is a carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive sub ...
; in fact, it is the second leading cause of lung cancer in the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country Continental United States, primarily located in North America. It consists of 50 U.S. state, states, a Washington, D.C., ...
after cigarette smoking
Tobacco smoking is the practice of burning tobacco and ingesting the resulting smoke. The smoke may be inhaled, as is done with cigarettes, or simply released from the mouth, as is generally done with pipes and cigars. The practice is believed ...
, with over 20,000 deaths per year attributed to radon-induced lung cancer.
See also
* Isotopes of radonNotes
References
{{reflist Isotopes of radon Carcinogens