Proton gradient on:
[Wikipedia]
[Google]
[Amazon]
 An electrochemical gradient is a gradient of
An electrochemical gradient is a gradient of
 Since the ions are charged, they cannot pass through cellular membranes via simple diffusion. Two different mechanisms can transport the ions across the membrane:
Since the ions are charged, they cannot pass through cellular membranes via simple diffusion. Two different mechanisms can transport the ions across the membrane:
 The way bacteriorhodopsin generates a proton gradient in Archaea is through a
The way bacteriorhodopsin generates a proton gradient in Archaea is through a
 PSII also relies on
PSII also relies on
 In the electron transport chain,
In the electron transport chain,
Stephen T. Abedon, "Important words and concepts from Chapter 8, Campbell & Reece, 2002 (1/14/2005)", for Biology 113 at the Ohio State University
{{DEFAULTSORT:Electrochemical Gradient Cellular respiration Electrochemical concepts Electrophysiology Membrane biology Physical quantities Thermodynamics
electrochemical potential
In electrochemistry, the electrochemical potential (ECP), ', is a thermodynamic measure of chemical potential that does not omit the energy contribution of electrostatics. Electrochemical potential is expressed in the unit of J/ mol.
Introductio ...
, usually for an ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
that can move across a membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. ...
. The gradient consists of two parts, the chemical gradient, or difference in solute concentration across a membrane, and the electrical gradient, or difference in charge
Charge or charged may refer to:
Arts, entertainment, and media Films
* '' Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* ''Charge!!'', an album by The Aqu ...
across a membrane. When there are unequal concentrations of an ion across a permeable membrane, the ion will move across the membrane from the area of higher concentration to the area of lower concentration through simple diffusion. Ions also carry an electric charge that forms an electric potential
The electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as the amount of work energy needed to move a unit of electric charge from a reference point to the specific point in ...
across a membrane. If there is an unequal distribution of charges across the membrane, then the difference in electric potential generates a force that drives ion diffusion until the charges are balanced on both sides of the membrane.
Electrochemical gradients are essential to the operation of batteries and other electrochemical cell
An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic o ...
s, photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
and cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
, and certain other biological processes.
Overview
Electrochemical energy is one of the many interchangeable forms of potential energy through which energy may be conserved. It appears in electroanalytical chemistry and has industrial applications such as batteries and fuel cells. In biology, electrochemical gradients allow cells to control the direction ions move across membranes. In mitochondria and chloroplasts, proton gradients generate a chemiosmotic potential used to synthesize ATP, and the sodium-potassium gradient helps neural synapses quickly transmit information. An electrochemical gradient has two components: a differential concentration ofelectric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respe ...
across a membrane and a differential concentration of chemical species
A chemical species is a chemical substance or ensemble composed of chemically identical molecular entities that can explore the same set of molecular energy levels on a characteristic or delineated time scale. These energy levels determine the wa ...
across that same membrane. In the former effect, the concentrated charge attracts charges of the opposite sign; in the latter, the concentrated species tends to diffuse across the membrane to an equalize concentrations. The combination of these two phenomena determines the thermodynamically-preferred direction for an ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
's movement across the membrane.
The combined effect can be quantified as a gradient in the thermodynamic electrochemical potential
In electrochemistry, the electrochemical potential (ECP), ', is a thermodynamic measure of chemical potential that does not omit the energy contribution of electrostatics. Electrochemical potential is expressed in the unit of J/ mol.
Introductio ...
: with - the chemical potential of the ion species
- the charge per ion of the species
- , Faraday constant In physical chemistry, the Faraday constant, denoted by the symbol and sometimes stylized as ℱ, is the electric charge per mole of elementary charges. It is named after the English scientist Michael Faraday. Since the 2019 redefinition of ...(the electrochemical potential is implicitly measured on a per-mole Mole (or Molé) may refer to: Animals * Mole (animal) or "true mole", mammals in the family Talpidae, found in Eurasia and North America * Golden moles, southern African mammals in the family Chrysochloridae, similar to but unrelated to Talpida ...basis)
- , the local electric potential The electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as the amount of work energy needed to move a unit of electric charge from a reference point to the specific point in ....
pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
across a hydroelectric dam
Hydroelectricity, or hydroelectric power, is electricity generated from hydropower (water power). Hydropower supplies one sixth of the world's electricity, almost 4500 TWh in 2020, which is more than all other renewable sources combined an ...
. Routes unblocked by the membrane (e.g. membrane transport protein
A membrane transport protein (or simply transporter) is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembra ...
or electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials d ...
s) correspond to turbines that convert the water's potential energy to other forms of physical or chemical energy, and the ions that pass through the membrane correspond to water traveling into the lower river. Conversely, energy can be used to pump water up into the lake above the dam, and chemical energy can be used to create electrochemical gradients.
Chemistry
The term typically applies inelectrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
, when electrical energy in the form of an applied voltage is used to modulate the thermodynamic favorability of a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
. In a battery, an electrochemical potential arising from the movement of ions balances the reaction energy of the electrodes. The maximum voltage that a battery reaction can produce is sometimes called the standard electrochemical potential of that reaction.
Biological context
The generation of a transmembrane electrical potential through ion movement across a cell membrane drives biological processes like nerve conduction,muscle contraction
Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as ...
, hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are require ...
secretion, and sensation. By convention, physiological voltages are measured relative to the extracellular regiond; a typical animal cell has an internal electrical potential of (−70)–(−50) mV.
An electrochemical gradient is essential to mitochondrial oxidative phosphorylation. The final step of cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
is the electron transport chain, composed of four complexes embedded in the inner mitochondrial membrane. Complexes I, III, and IV pump protons from the matrix to the intermembrane space (IMS); for every electron pair entering the chain, ten protons translocate into the IMS. The result is an electric potential of more than . The resulting flux of protons back into the matrix powers the efforts of ATP synthase to combine inorganic phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
and ADP.
Similar to the electron transport chain, the light-dependent reactions of photosynthesis pump protons into the thylakoid lumen of chloroplasts to drive the synthesis of ATP. The proton gradient can be generated through either noncyclic or cyclic photophosphorylation. Of the proteins that participate in noncyclic photophosphorylation, photosystem II (PSII), plastiquinone, and cytochrome b6f complex directly contribute to generating the proton gradient. For each four photons absorbed by PSII, eight protons are pumped into the lumen.
Several other transporters and ion channels play a role in generating a proton electrochemical gradient. One is TPK3, a potassium channel
Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of c ...
that is activated by Ca2+ and conducts K+ from the thylakoid lumen to the stroma, which helps establish the electric field. On the other hand, the electro-neutral K+ efflux antiporter (KEA3) transports K+ into the thylakoid lumen and H+ into the stroma, which helps establish the pH gradient.
Ion gradients
active
Active may refer to:
Music
* ''Active'' (album), a 1992 album by Casiopea
* Active Records, a record label
Ships
* ''Active'' (ship), several commercial ships by that name
* HMS ''Active'', the name of various ships of the British Royal ...
or passive
Passive may refer to:
* Passive voice, a grammatical voice common in many languages, see also Pseudopassive
* Passive language, a language from which an interpreter works
* Passivity (behavior), the condition of submitting to the influence of o ...
transport.
An example of active transport of ions is the Na+-K+-ATPase (NKA). NKA is powered by the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
of ATP into ADP and an inorganic phosphate; for every molecule of ATP hydrolized, three Na+ are transported outside and two K+ are transported inside the cell. This makes the inside of the cell more negative than the outside and more specifically generates a membrane potential ''V''membrane of about .
An example of passive transport is ion fluxes through Na+, K+, Ca2+, and Cl− channels. Unlike active transport, passive transport is powered by the arithmetic sum of osmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region o ...
(a concentration gradient) and an electric field (the transmembrane potential). Formally, the molar Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and ...
change associated with successful transport is where represents the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
, represents absolute temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic w ...
, is the charge per ion, and represents the Faraday constant
In physical chemistry, the Faraday constant, denoted by the symbol and sometimes stylized as ℱ, is the electric charge per mole of elementary charges. It is named after the English scientist Michael Faraday. Since the 2019 redefinition of ...
.
In the example of Na+, both terms tend to support transport: the negative electric potential inside the cell attracts the positive ion and since Na+ is concentrated outside the cell, osmosis supports diffusion through the Na+ channel into the cell. In the case of K+, the effect of osmosis is reversed: although external ions are attracted by the negative intracellular potential, entropy seeks to diffuse the ions already concentrated inside the cell. The converse phenomenon (osmosis supports transport, electric potential opposes it) can be achieved for Na+ in cells with abnormal transmembrane potentials: at , the Na+ influx halts; at higher potentials, it becomes an efflux.
Proton gradients
Proton gradients in particular are important in many types of cells as a form of energy storage. The gradient is usually used to drive ATP synthase, flagellar rotation, or metabolite transport. This section will focus on three processes that help establish proton gradients in their respective cells: bacteriorhodopsin and noncyclic photophosphorylation and oxidative phosphorylation.Bacteriorhodopsin
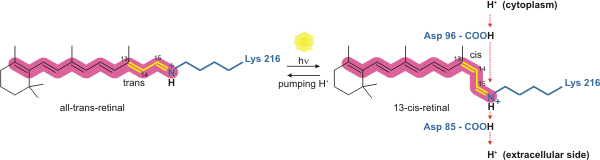
 The way bacteriorhodopsin generates a proton gradient in Archaea is through a
The way bacteriorhodopsin generates a proton gradient in Archaea is through a proton pump
A proton pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane
A biological membrane, biomembrane or cell membrane is a selectively permeable membrane that separates the interior of a cell f ...
. The proton pump relies on proton carriers to drive protons from the side of the membrane with a low H+ concentration to the side of the membrane with a high H+ concentration. In bacteriorhodopsin, the proton pump is activated by absorption of photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they a ...
s of 568 nm wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, t ...
which leads to isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
of the Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimine ...
(SB) in retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
Some microorganisms use reti ...
forming the K state. This moves SB away from Asp85 and Asp212, causing H+ transfer from the SB to Asp85 forming the M1 state. The protein then shifts to the M2 state by separating Glu204 from Glu194 which releases a proton from Glu204 into the external medium. The SB is reprotonated by Asp96 which forms the N state. It is important that the second proton comes from Asp96 since its deprotonated
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
state is unstable and rapidly reprotonated with a proton from the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
. The protonation of Asp85 and Asp96 causing re-isomerization of the SB forming the O state. Finally, bacteriorhodopsin returns to its resting state when Asp85 releases its proton to Glu204.
Photophosphorylation
light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
to drive the formation of proton gradients in chloroplasts, however PSII utilizes vectorial redox chemistry to achieve this goal. Rather than physically transporting protons through the protein, reactions requiring the binding of protons will occur on the extracellular side while reactions requiring the release of protons will occur on the intracellular side. Absorption of photons of 680 nm wavelength is used to excite two electrons in P680 to a higher energy level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The t ...
. These higher energy electrons are transferred to protein-bound plastoquinone (PQA) and then to unbound plastoquinone (PQB). This reduces plastoquinone (PQ) to plastoquinol (PQH2) which is released from PSII after gaining two protons from the stroma. The electrons in P680 are replenished by oxidizing water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
through the oxygen-evolving complex
The oxygen-evolving complex (OEC), also known as the water-splitting complex, is the portion of photosystem II where photo-oxidation of water occurs during the light reactions of photosynthesis. The OEC is surrounded by four core protein subunits ...
(OEC). This results in release of O2 and H+ into the lumen, for a total reaction of
After being released from PSII, PQH2 travels to the cytochrome b6f complex which then transfers two electrons from PQH2 to plastocyanin
Plastocyanin is a copper-containing protein that mediates electron-transfer. It is found in a variety of plants, where it participates in photosynthesis. The protein is a prototype of the blue copper proteins, a family of intensely blue-colored ...
in two separate reactions. The process that occurs is similar to the Q-cycle in Complex III of the electron transport chain. In the first reaction, PQH2 binds to the complex on the lumen side and one electron is transferred to the iron-sulfur center which then transfers it to cytochrome f
Cytochrome ''f'' is the largest subunit of cytochrome ''b''6''f'' complex (plastoquinol—plastocyanin reductase; ). In its structure and functions, the cytochrome b6f complex bears extensive analogy to the cytochrome bc1 complex of mitochondr ...
which then transfers it to plastocyanin. The second electron is transferred to heme bL which then transfers it to heme bH which then transfers it to PQ. In the second reaction, a second PQH2 gets oxidized, adding an electron to another plastocyanin and PQ. Both reactions together transfer four protons into the lumen.
Oxidative phosphorylation
complex I
Respiratory complex I, (also known as NADH:ubiquinone oxidoreductase, Type I NADH dehydrogenase and mitochondrial complex I) is the first large protein complex of the respiratory chains of many organisms from bacteria to humans. It catalyzes the ...
(CI) catalyzes the reduction of ubiquinone
Coenzyme Q, also known as ubiquinone and marketed as CoQ10, is a coenzyme family that is ubiquitous in animals and most bacteria (hence the name ubiquinone). In humans, the most common form is coenzyme Q10 or ubiquinone-10.
It is a 1,4-benzoq ...
(UQ) to ubiquinol (UQH2) by the transfer of two electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
s from reduced nicotinamide adenine dinucleotide (NADH) which translocates four protons from the mitochondrial matrix to the IMS:
Complex III
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
(CIII) catalyzes the Q-cycle. The first step involving the transfer of two electrons from the UQH2 reduced by CI to two molecules of oxidized cytochrome c at the Qo site. In the second step, two more electrons reduce UQ to UQH2 at the Qi site. The total reaction is:
Complex IV (CIV) catalyzes the transfer of two electrons from the cytochrome c reduced by CIII to one half of a full oxygen. Utilizing one full oxygen in oxidative phosphorylation requires the transfer of four electrons. The oxygen will then consume four protons from the matrix to form water while another four protons are pumped into the IMS, to give a total reaction
See also
*Concentration cell
In battery technology, a concentration cell is a limited form of a galvanic cell that has two equivalent half-cells of the same composition differing only in concentrations. One can calculate the potential developed by such a cell using the Nernst ...
* Transmembrane potential difference
*Action potential
An action potential occurs when the membrane potential of a specific cell location rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, ...
* Cell potential
*Electrodiffusion
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of ...
*Galvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus ...
*Electrochemical cell
An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic o ...
* Proton exchange membrane
*Reversal potential In a biological membrane, the reversal potential is the membrane potential at which the direction of ionic current reverses. At the reversal potential, there is no net flow of ions from one side of the membrane to the other. For channels that are pe ...
References
*Stephen T. Abedon, "Important words and concepts from Chapter 8, Campbell & Reece, 2002 (1/14/2005)", for Biology 113 at the Ohio State University
{{DEFAULTSORT:Electrochemical Gradient Cellular respiration Electrochemical concepts Electrophysiology Membrane biology Physical quantities Thermodynamics