Polyacrylamide gel electrophoresis on:
[Wikipedia]
[Google]
[Amazon]
 Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in
Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in
 The sample to analyze is optionally mixed with a chemical denaturant if so desired, usually SDS for proteins or urea for nucleic acids. SDS is an anionic
The sample to analyze is optionally mixed with a chemical denaturant if so desired, usually SDS for proteins or urea for nucleic acids. SDS is an anionic 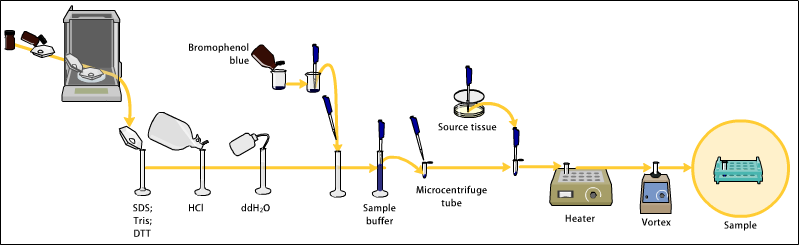
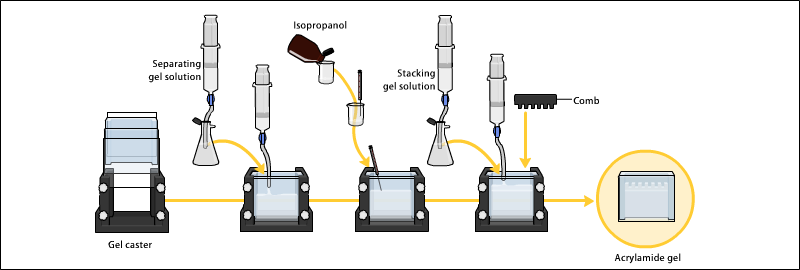
 Gels are usually polymerized between two glass plates in a gel caster, with a comb inserted at the top to create the sample wells. After the gel is polymerized the comb can be removed and the gel is ready for electrophoresis.
Gels are usually polymerized between two glass plates in a gel caster, with a comb inserted at the top to create the sample wells. After the gel is polymerized the comb can be removed and the gel is ready for electrophoresis.

 An electric field is applied across the gel, causing the negatively charged proteins or nucleic acids to migrate across the gel away from the negative electrode (which is the cathode being that this is an electrolytic rather than galvanic cell) and towards the positive electrode (the anode). Depending on their size, each biomolecule moves differently through the gel matrix: small molecules more easily fit through the pores in the gel, while larger ones have more difficulty. The gel is run usually for a few hours, though this depends on the voltage applied across the gel; migration occurs more quickly at higher voltages, but these results are typically less accurate than at those at lower voltages. After the set amount of time, the biomolecules have migrated different distances based on their size. Smaller biomolecules travel farther down the gel, while larger ones remain closer to the point of origin. Biomolecules may therefore be separated roughly according to size, which depends mainly on molecular weight under denaturing conditions, but also depends on higher-order conformation under native conditions. The gel mobility is defined as the rate of migration traveled with a voltage gradient of 1V/cm and has units of cm2/sec/V. For analytical purposes, the relative mobility of biomolecules, ''Rf'', the ratio of the distance the molecule traveled on the gel to the total travel distance of a tracking dye is plotted versus the molecular weight of the molecule (or sometimes the log of MW, or rather the Mr, molecular radius). Such typically linear plots represent the standard markers or calibration curves that are widely used for the quantitative estimation of a variety of biomolecular sizes.
Certain
An electric field is applied across the gel, causing the negatively charged proteins or nucleic acids to migrate across the gel away from the negative electrode (which is the cathode being that this is an electrolytic rather than galvanic cell) and towards the positive electrode (the anode). Depending on their size, each biomolecule moves differently through the gel matrix: small molecules more easily fit through the pores in the gel, while larger ones have more difficulty. The gel is run usually for a few hours, though this depends on the voltage applied across the gel; migration occurs more quickly at higher voltages, but these results are typically less accurate than at those at lower voltages. After the set amount of time, the biomolecules have migrated different distances based on their size. Smaller biomolecules travel farther down the gel, while larger ones remain closer to the point of origin. Biomolecules may therefore be separated roughly according to size, which depends mainly on molecular weight under denaturing conditions, but also depends on higher-order conformation under native conditions. The gel mobility is defined as the rate of migration traveled with a voltage gradient of 1V/cm and has units of cm2/sec/V. For analytical purposes, the relative mobility of biomolecules, ''Rf'', the ratio of the distance the molecule traveled on the gel to the total travel distance of a tracking dye is plotted versus the molecular weight of the molecule (or sometimes the log of MW, or rather the Mr, molecular radius). Such typically linear plots represent the standard markers or calibration curves that are widely used for the quantitative estimation of a variety of biomolecular sizes.
Certain
 Following electrophoresis, the gel may be stained (for proteins, most commonly with Coomassie brilliant blue R-250 or autoradiography; for nucleic acids,
Following electrophoresis, the gel may be stained (for proteins, most commonly with Coomassie brilliant blue R-250 or autoradiography; for nucleic acids,
 The following chemicals and procedures are used for processing of the gel and the protein samples visualized in it.
Tracking dye; as proteins and nucleic acids are mostly colorless, their progress through the gel during electrophoresis cannot be easily followed. Anionic dyes of a known electrophoretic mobility are therefore usually included in the PAGE sample buffer. A very common tracking dye is
The following chemicals and procedures are used for processing of the gel and the protein samples visualized in it.
Tracking dye; as proteins and nucleic acids are mostly colorless, their progress through the gel during electrophoresis cannot be easily followed. Anionic dyes of a known electrophoretic mobility are therefore usually included in the PAGE sample buffer. A very common tracking dye is
SDS-PAGE: How it Works
Demystifying SDS-PAGE
for customised recipes for TRIS Urea gels.
2-Dimensional Protein Gelelectrophoresis
Hempelmann E. SDS-Protein PAGE and Proteindetection by Silverstaining and Immunoblotting of Plasmodium falciparum proteins. in: Moll K, Ljungström J, Perlmann H, Scherf A, Wahlgren M (eds) Methods in Malaria Research, 5th edition, 2008, 263-266 {{DEFAULTSORT:Sds-Page Molecular biology Electrophoresis
 Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in
Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, forensic chemistry
Forensic chemistry is the application of chemistry and its subfield, forensic toxicology, in a legal setting. A forensic chemist can assist in the identification of unknown materials found at a crime scene. Specialists in this field have a wid ...
, genetics
Genetics is the study of genes, genetic variation, and heredity in organisms.Hartl D, Jones E (2005) It is an important branch in biology because heredity is vital to organisms' evolution. Gregor Mendel, a Moravian Augustinian friar work ...
, molecular biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and phys ...
and biotechnology
Biotechnology is the integration of natural sciences and engineering sciences in order to achieve the application of organisms, cells, parts thereof and molecular analogues for products and services. The term ''biotechnology'' was first used ...
to separate biological macromolecule
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
s, usually protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s or nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main ...
s, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation, and charge of the molecule. Polyacrylamide
Polyacrylamide (abbreviated as PAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly fo ...
gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.
Hydration Hydration may refer to:
* Hydrate, a substance that contains water
* Hydration enthalpy, energy released through hydrating a substance
* Hydration reaction, a chemical addition reaction where a hydroxyl group and proton are added to a compound
* ...
of acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecula ...
results in formation of acrylamide
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. From the chemistry perspective, acrylamide is a vinyl-substituted primar ...
molecules () by nitrile hydratase
Nitrile hydratases (NHases; ) are mononuclear iron or non-corrinoid cobalt enzymes that catalyse the hydration of diverse nitriles to their corresponding amides
R-C≡N + H2O → R-C(O)NH2
Metal cofactor
In biochemistry, cobalt is in genera ...
. Acrylamide monomer is in a powder state before addition of water. Acrylamide is toxic to the human nervous system, therefore all safety measures must be followed when working with it. Acrylamide is soluble in water and upon addition of free-radical initiators it polymerizes resulting in formation of polyacrylamide. It is useful to make polyacrylamide gel via acrylamide hydration because pore size can be regulated. Increased concentrations of acrylamide result in decreased pore size after polymerization. Polyacrylamide gel with small pores helps to examine smaller molecules better since the small molecules can enter the pores and travel through the gel while large molecules get trapped at the pore openings.
As with all forms of gel electrophoresis
Gel electrophoresis is a method for separation and analysis of biomacromolecules ( DNA, RNA, proteins, etc.) and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size (IEF ...
, molecules may be run in their native state
In biochemistry, the native state of a protein or nucleic acid is its properly folded and/or assembled form, which is operative and functional. The native state of a biomolecule may possess all four levels of biomolecular structure, with the ...
, preserving the molecules' higher-order structure. This method is called native-PAGE. Alternatively, a chemical denaturant may be added to remove this structure and turn the molecule into an unstructured molecule whose mobility depends only on its length (because the protein-SDS complexes all have a similar mass-to-charge ratio). This procedure is called SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a Discontinuous electrophoresis, discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular m ...
. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is a method of separating molecules based on the difference of their molecular weight. At the pH at which gel electrophoresis is carried out the SDS molecules are negatively charged and bind to proteins in a set ratio, approximately one molecule of SDS for every 2 amino acids. In this way, the detergent provides all proteins with a uniform charge-to-mass ratio. By binding to the proteins the detergent destroys their secondary, tertiary and/or quaternary structure denaturing them and turning them into negatively charged linear polypeptide chains. When subjected to an electric field in PAGE, the negatively charged polypeptide chains travel toward the anode with different mobility. Their mobility, or the distance traveled by molecules, is inversely proportional to the logarithm of their molecular weight. By comparing the relative ratio of the distance traveled by each protein to the length of the gel (Rf) one can make conclusions about the relative molecular weight of the proteins, where the length of the gel is determined by the distance traveled by a small molecule like a tracking dye.
For nucleic acids, urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
is the most commonly used denaturant. For proteins, sodium dodecyl sulfate
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula . It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt ...
(SDS) is an anionic detergent applied to protein samples to coat proteins in order to impart two negative charges (from every SDS molecule) to every two amino acids of the denatured protein. 2-Mercaptoethanol
2-Mercaptoethanol (also β-mercaptoethanol, BME, 2BME, 2-ME or β-met) is the chemical compound with the formula HOCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant by s ...
may also be used to disrupt the disulfide bonds found between the protein complexes, which helps further denature the protein. In most proteins, the binding of SDS to the polypeptide chains impart an even distribution of charge per unit mass, thereby resulting in a fractionation by approximate size during electrophoresis. Proteins that have a greater hydrophobic content – for instance, many membrane proteins, and those that interact with surfactants in their native environment – are intrinsically harder to treat accurately using this method, due to the greater variability in the ratio of bound SDS. Procedurally, using both Native and SDS-PAGE together can be used to purify and to separate the various subunits of the protein. Native-PAGE keeps the oligomeric form intact and will show a band on the gel that is representative of the level of activity. SDS-PAGE will denature and separate the oligomeric form into its monomers, showing bands that are representative of their molecular weights. These bands can be used to identify and assess the purity of the protein.
Procedure
Sample preparation
Samples may be any material containing proteins or nucleic acids. These may be biologically derived, for example from prokaryotic or eukaryotic cells, tissues, viruses, environmental samples, or purified proteins. In the case of solid tissues or cells, these are often first broken down mechanically using ablender
A blender (sometimes called a mixer or liquidiser in British English) is a kitchen and laboratory appliance used to mix, crush, purée or emulsify food and other substances. A stationary blender consists of a blender container with a rotating me ...
(for larger sample volumes), using a homogenizer (smaller volumes), by sonicator
image:Sonicator.jpg, A sonicator at the Weizmann Institute of Science during sonicationSonication is the act of applying sound energy to agitate particles in a sample, for various purposes such as the extraction of multiple compounds from plants, m ...
or by using cycling of high pressure, and a combination of biochemical and mechanical techniques – including various types of filtration and centrifugation
Centrifugation is a mechanical process which involves the use of the centrifugal force to separate particles from a solution according to their size, shape, density, medium viscosity and rotor speed. The denser components of the mixture migrate ...
– may be used to separate different cell compartments and organelle
In cell biology, an organelle is a specialized subunit, usually within a cell, that has a specific function. The name ''organelle'' comes from the idea that these structures are parts of cells, as organs are to the body, hence ''organelle,'' th ...
s prior to electrophoresis. Synthetic biomolecules such as oligonucleotides may also be used as analytes.
 The sample to analyze is optionally mixed with a chemical denaturant if so desired, usually SDS for proteins or urea for nucleic acids. SDS is an anionic
The sample to analyze is optionally mixed with a chemical denaturant if so desired, usually SDS for proteins or urea for nucleic acids. SDS is an anionic detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are m ...
that denatures secondary and non–disulfide–linked tertiary structures, and additionally applies a negative charge to each protein in proportion to its mass. Urea breaks the hydrogen bonds between the base pair
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both D ...
s of the nucleic acid, causing the constituent strands to separate. Heating the samples to at least 60 °C further promotes denaturation.
In addition to SDS, proteins may optionally be briefly heated to near boiling in the presence of a reducing agent, such as dithiothreitol (DTT) or 2-mercaptoethanol (beta-mercaptoethanol/BME), which further denatures the proteins by reducing disulfide linkages, thus overcoming some forms of tertiary protein folding, and breaking up quaternary protein structure (oligomeric subunits). This is known as reducing SDS-PAGE.
A tracking dye may be added to the solution. This typically has a higher electrophoretic mobility than the analytes to allow the experimenter to track the progress of the solution through the gel during the electrophoretic run.

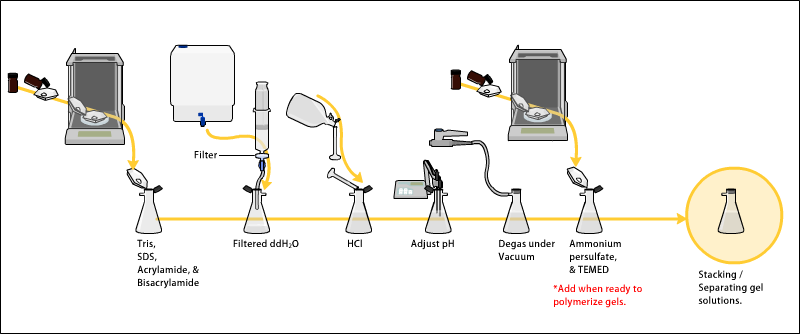
Preparing acrylamide gels
The gels typically consist ofacrylamide
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. From the chemistry perspective, acrylamide is a vinyl-substituted primar ...
, bisacrylamide, the optional denaturant (SDS or urea), and a buffer with an adjusted pH. The solution may be degassed under a vacuum to prevent the formation of air bubbles during polymerization. Alternatively, butanol may be added to the resolving gel (for proteins) after it is poured, as butanol removes bubbles and makes the surface smooth.
A source of free radicals and a stabilizer, such as ammonium persulfate and TEMED are added to initiate polymerization. The polymerization reaction creates a gel because of the added bisacrylamide, which can form cross-links between two acrylamide molecules. The ratio of bisacrylamide to acrylamide can be varied for special purposes, but is generally about 1 part in 35. The acrylamide concentration of the gel can also be varied, generally in the range from 5% to 25%. Lower percentage gels are better for resolving very high molecular weight molecules, while much higher percentages of acrylamide are needed to resolve smaller proteins. The average pore diameter of polyacrylamide gels is determined by the total concentration of acrylamides (% T with T = Total concentration of acrylamide and bisacrylamide) and the concentration of the cross-linker
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
bisacrylamide (%C with C = bisacrylamide concentration). The pore size is reduced reciprocally to the %T. Concerning %C, a concentration of 5% produces the smallest pores, since the influence of bisacrylamide on the pore size has a parabola
In mathematics, a parabola is a plane curve which is mirror-symmetrical and is approximately U-shaped. It fits several superficially different mathematical descriptions, which can all be proved to define exactly the same curves.
One descri ...
-shape with a vertex
Vertex, vertices or vertexes may refer to:
Science and technology Mathematics and computer science
*Vertex (geometry), a point where two or more curves, lines, or edges meet
*Vertex (computer graphics), a data structure that describes the position ...
at 5%.
 Gels are usually polymerized between two glass plates in a gel caster, with a comb inserted at the top to create the sample wells. After the gel is polymerized the comb can be removed and the gel is ready for electrophoresis.
Gels are usually polymerized between two glass plates in a gel caster, with a comb inserted at the top to create the sample wells. After the gel is polymerized the comb can be removed and the gel is ready for electrophoresis.

Electrophoresis
Various buffer systems are used in PAGE depending on the nature of the sample and the experimental objective. The buffers used at the anode and cathode may be the same or different.
 An electric field is applied across the gel, causing the negatively charged proteins or nucleic acids to migrate across the gel away from the negative electrode (which is the cathode being that this is an electrolytic rather than galvanic cell) and towards the positive electrode (the anode). Depending on their size, each biomolecule moves differently through the gel matrix: small molecules more easily fit through the pores in the gel, while larger ones have more difficulty. The gel is run usually for a few hours, though this depends on the voltage applied across the gel; migration occurs more quickly at higher voltages, but these results are typically less accurate than at those at lower voltages. After the set amount of time, the biomolecules have migrated different distances based on their size. Smaller biomolecules travel farther down the gel, while larger ones remain closer to the point of origin. Biomolecules may therefore be separated roughly according to size, which depends mainly on molecular weight under denaturing conditions, but also depends on higher-order conformation under native conditions. The gel mobility is defined as the rate of migration traveled with a voltage gradient of 1V/cm and has units of cm2/sec/V. For analytical purposes, the relative mobility of biomolecules, ''Rf'', the ratio of the distance the molecule traveled on the gel to the total travel distance of a tracking dye is plotted versus the molecular weight of the molecule (or sometimes the log of MW, or rather the Mr, molecular radius). Such typically linear plots represent the standard markers or calibration curves that are widely used for the quantitative estimation of a variety of biomolecular sizes.
Certain
An electric field is applied across the gel, causing the negatively charged proteins or nucleic acids to migrate across the gel away from the negative electrode (which is the cathode being that this is an electrolytic rather than galvanic cell) and towards the positive electrode (the anode). Depending on their size, each biomolecule moves differently through the gel matrix: small molecules more easily fit through the pores in the gel, while larger ones have more difficulty. The gel is run usually for a few hours, though this depends on the voltage applied across the gel; migration occurs more quickly at higher voltages, but these results are typically less accurate than at those at lower voltages. After the set amount of time, the biomolecules have migrated different distances based on their size. Smaller biomolecules travel farther down the gel, while larger ones remain closer to the point of origin. Biomolecules may therefore be separated roughly according to size, which depends mainly on molecular weight under denaturing conditions, but also depends on higher-order conformation under native conditions. The gel mobility is defined as the rate of migration traveled with a voltage gradient of 1V/cm and has units of cm2/sec/V. For analytical purposes, the relative mobility of biomolecules, ''Rf'', the ratio of the distance the molecule traveled on the gel to the total travel distance of a tracking dye is plotted versus the molecular weight of the molecule (or sometimes the log of MW, or rather the Mr, molecular radius). Such typically linear plots represent the standard markers or calibration curves that are widely used for the quantitative estimation of a variety of biomolecular sizes.
Certain glycoprotein
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glyco ...
s, however, behave anomalously on SDS gels. Additionally, the analysis of larger proteins ranging from 250,000 to 600,000 Da is also reported to be problematic due to the fact that such polypeptides move improperly in the normally used gel systems.
Further processing
 Following electrophoresis, the gel may be stained (for proteins, most commonly with Coomassie brilliant blue R-250 or autoradiography; for nucleic acids,
Following electrophoresis, the gel may be stained (for proteins, most commonly with Coomassie brilliant blue R-250 or autoradiography; for nucleic acids, ethidium bromide
Ethidium bromide (or homidium bromide, chloride salt homidium chloride) is an intercalating agent commonly used as a fluorescent tag ( nucleic acid stain) in molecular biology laboratories for techniques such as agarose gel electrophoresis. It ...
; or for either, silver stain), allowing visualization of the separated proteins, or processed further (e.g. Western blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
). After staining, different species biomolecules appear as distinct bands within the gel. It is common to run molecular weight size markers of known molecular weight in a separate lane in the gel to calibrate the gel and determine the approximate molecular mass
The molecular mass (''m'') is the mass of a given molecule: it is measured in daltons (Da or u). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The related quant ...
of unknown biomolecules by comparing the distance traveled relative to the marker.
For proteins, SDS-PAGE is usually the first choice as an assay of purity due to its reliability and ease. The presence of SDS and the denaturing step make proteins separate, approximately based on size, but aberrant migration of some proteins may occur. Different proteins may also stain differently, which interferes with quantification by staining. PAGE may also be used as a preparative technique for the purification of proteins. For example, quantitative preparative native continuous polyacrylamide gel electrophoresis ( QPNC-PAGE) is a method for separating native metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains al ...
s in complex biological matrices.
Chemical ingredients and their roles
''Polyacrylamide gel (PAG)'' had been known as a potential embedding medium for sectioning tissues as early as 1964, and two independent groups employed PAG in electrophoresis in 1959. It possesses several electrophoretically desirable features that make it a versatile medium. It is a synthetic, thermo-stable, transparent, strong, chemically relatively inert gel, and can be prepared with a wide range of average pore sizes. The pore size of a gel and the reproducibility in gel pore size are determined by three factors, the total amount of acrylamide present (%T) (T = Total concentration of acrylamide and bisacrylamide monomer), the amount of cross-linker (%C) (C = bisacrylamide concentration), and the time of polymerization of acrylamide (cf. QPNC-PAGE). Pore size decreases with increasing %T; with cross-linking, 5%C gives the smallest pore size. Any increase or decrease in %C from 5% increases the pore size, as pore size with respect to %C is a parabolic function with vertex as 5%C. This appears to be because of non-homogeneous bundling of polymer strands within the gel. This gel material can also withstand highvoltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to ...
gradients, is amenable to various staining and destaining procedures, and can be digested to extract separated fractions or dried for autoradiography
An autoradiograph is an image on an X-ray film or nuclear emulsion produced by the pattern of decay emissions (e.g., beta particles or gamma rays) from a distribution of a radioactive substance. Alternatively, the autoradiograph is also available ...
and permanent recording.
Components
Polyacrylamide gels are composed of a stacking gel and separating gel. Stacking gels have a higher porosity relative to the separating gel, and allow for proteins to migrate in a concentrated area. Additionally, stacking gels usually have a pH of 6.8, since the neutral glycine molecules allow for faster protein mobility. Separating gels have a pH of 8.8, where the anionic glycine slows down the mobility of proteins. Separating gels allow for the separation of proteins and have a relatively lower porosity. Here, the proteins are separated based on size (in SDS-PAGE) and size/ charge (Native PAGE). Chemical buffer stabilizes the pH value to the desired value within the gel itself and in the electrophoresis buffer. The choice of buffer also affects the electrophoretic mobility of the buffercounterion
160px, Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.">cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typical ...
s and thereby the resolution of the gel. The buffer should also be unreactive and not modify or react with most proteins. Different buffers may be used as cathode and anode buffers, respectively, depending on the application. Multiple pH values may be used within a single gel, for example in DISC electrophoresis. Common buffers in PAGE include Tris
Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2, one of the twenty Good's buffers. It is extensively used in biochemistry and molecular biology as ...
, Bis-Tris, or imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non ...
.
Counterion
160px, Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.">cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typical ...
balance the intrinsic charge of the buffer ion and also affect the electric field strength during electrophoresis. Highly charged and mobile ions are often avoided in SDS-PAGE cathode buffers, but may be included in the gel itself, where it migrates ahead of the protein. In applications such as DISC SDS-PAGE the pH values within the gel may vary to change the average charge of the counterions during the run to improve resolution. Popular counterions are glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
and tricine. Glycine has been used as the source of trailing ion or slow ion because its pKa is 9.69 and mobility of glycinate are such that the effective mobility can be set at a value below that of the slowest known proteins of net negative charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectiv ...
in the pH range. The minimum pH of this range is approximately 8.0.
Acrylamide
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. From the chemistry perspective, acrylamide is a vinyl-substituted primar ...
(; mW: 71.08) when dissolved in water, slow, spontaneous autopolymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many f ...
of acrylamide takes place, joining molecules together by head on tail fashion to form long single-chain polymers. The presence of a free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
-generating system greatly accelerates polymerization. This kind of reaction is known as vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
addition polymerisation. A solution of these polymer chains becomes viscous but does not form a gel, because the chains simply slide over one another. Gel formation requires linking various chains together. Acrylamide is carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive sub ...
ic, a neurotoxin
Neurotoxins are toxins that are destructive to nerve tissue (causing neurotoxicity). Neurotoxins are an extensive class of exogenous chemical neurological insultsSpencer 2000 that can adversely affect function in both developing and mature nerv ...
, and a reproductive toxin. It is also essential to store acrylamide in a cool dark and dry place to reduce autopolymerisation and hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
.
Bisacrylamide ( ''N'',''N''′-Methylenebisacrylamide) (; mW: 154.17) is the most frequently used cross linking agent for polyacrylamide gels. Chemically it can be thought of as two acrylamide molecules coupled head to head at their non-reactive ends. Bisacrylamide can crosslink two polyacrylamide chains to one another, thereby resulting in a gel.
Sodium dodecyl sulfate
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula . It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt ...
(SDS) (; mW: 288.38) (only used in denaturing protein gels) is a strong detergent agent used to denature native proteins to individual polypeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
s. This denaturation, which is referred to as reconstructive denaturation, is not accomplished by the total linearization of the protein, but instead, through a conformational change to a combination of random coil and α helix secondary structures. When a protein mixture is heated to 100 °C in presence of SDS, the detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are m ...
wraps around the polypeptide backbone. It binds to polypeptides in a constant weight ratio of 1.4 g SDS/g of polypeptide. In this process, the intrinsic charges of polypeptides become negligible when compared to the negative charges contributed by SDS. Thus polypeptides after treatment become rod-like structures possessing a uniform charge density, that is same net negative charge per unit weight. The electrophoretic mobilities of these proteins is a linear function of the logarithm
In mathematics, the logarithm is the inverse function to exponentiation. That means the logarithm of a number to the base is the exponent to which must be raised, to produce . For example, since , the ''logarithm base'' 10 ...
s of their molecular weights. Without SDS, different proteins with similar molecular weights would migrate differently due to differences in mass-charge ratio, as each protein has an isoelectric point
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also ...
and molecular weight particular to its primary structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynth ...
. This is known as native PAGE. Adding SDS solves this problem, as it binds to and unfolds the protein, giving a near uniform negative charge along the length of the polypeptide.
Urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
(; mW: 60.06) is a chaotropic agent that increases the entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
of the system by interfering with intramolecular interactions mediated by non-covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
forces such as hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
s and van der Waals forces
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
. Macromolecular structure is dependent on the net effect of these forces, therefore it follows that an increase in chaotropic solutes denatures macromolecules,
Ammonium persulfate (APS) (; mW: 228.2) is a source of free radicals and is often used as an initiator for gel formation. An alternative source of free radicals is riboflavin
Riboflavin, also known as vitamin B2, is a vitamin found in food and sold as a dietary supplement. It is essential to the formation of two major coenzymes, flavin mononucleotide and flavin adenine dinucleotide. These coenzymes are involved i ...
, which generated free radicals in a photochemical reaction.
TEMED (''N'', ''N'', ''N''′, ''N''′-tetramethylethylenediamine) (; mW: 116.21) stabilizes free radicals and improves polymerization. The rate of polymerisation and the properties of the resulting gel depend on the concentrations of free radicals. Increasing the amount of free radicals results in a decrease in the average polymer chain length, an increase in gel turbidity and a decrease in gel elasticity. Decreasing the amount shows the reverse effect. The lowest catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
concentrations that allow polymerisation in a reasonable period of time should be used. APS and TEMED are typically used at approximately equimolar concentrations in the range of 1 to 10 mM.
Chemicals for processing and visualization
 The following chemicals and procedures are used for processing of the gel and the protein samples visualized in it.
Tracking dye; as proteins and nucleic acids are mostly colorless, their progress through the gel during electrophoresis cannot be easily followed. Anionic dyes of a known electrophoretic mobility are therefore usually included in the PAGE sample buffer. A very common tracking dye is
The following chemicals and procedures are used for processing of the gel and the protein samples visualized in it.
Tracking dye; as proteins and nucleic acids are mostly colorless, their progress through the gel during electrophoresis cannot be easily followed. Anionic dyes of a known electrophoretic mobility are therefore usually included in the PAGE sample buffer. A very common tracking dye is Bromophenol blue
Bromophenol blue (3′,3″,5′,5″-tetrabromophenolsulfonphthalein, BPB), albutest is used as a pH indicator, an electrophoretic color marker, and a dye. It can be prepared by slowly adding excess bromine to a hot solution of phenolsulfonp ...
(BPB, 3',3",5',5" tetrabromophenolsulfonphthalein). This dye is coloured at alkali and neutral pH and is a small negatively charged molecule that moves towards the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
. Being a highly mobile molecule it moves ahead of most proteins. As it reaches the anodic end of the electrophoresis medium electrophoresis is stopped. It can weakly bind to some proteins and impart a blue colour. Other common tracking dyes are xylene cyanol
Xylene cyanol can be used as an electrophoretic color marker, or tracking dye, to monitor the process of agarose gel electrophoresis and polyacrylamide gel electrophoresis. Bromophenol blue
Bromophenol blue (3′,3″,5′,5″-tetrabromophen ...
, which has lower mobility, and Orange G
Orange G also called C.I. 16230, Acid Orange 10, or orange gelb is a synthetic azo dye used in histology in many staining formulations. It usually comes as a disodium salt. It has the appearance of orange crystals or powder.
Staining
Orange G ...
, which has a higher mobility.
Loading aids; most PAGE systems are loaded from the top into wells within the gel. To ensure that the sample sinks to the bottom of the gel, sample buffer is supplemented with additives that increase the density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematicall ...
of the sample. These additives should be non-ionic and non-reactive towards proteins to avoid interfering with electrophoresis. Common additives are glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
and sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refine ...
.
Coomassie brilliant blue R-250 (CBB)(; mW: 825.97) is the most popular protein stain. It is an anionic dye, which non-specifically binds to proteins. The structure of CBB is predominantly non-polar, and it is usually used in methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
ic solution acidified with acetic acid. Proteins in the gel are fixed by acetic acid and simultaneously stained. The excess dye incorporated into the gel can be removed by destaining with the same solution without the dye. The proteins are detected as blue bands on a clear background. As SDS is also anionic, it may interfere with staining process. Therefore, large volume of staining solution is recommended, at least ten times the volume of the gel.
Ethidium bromide
Ethidium bromide (or homidium bromide, chloride salt homidium chloride) is an intercalating agent commonly used as a fluorescent tag ( nucleic acid stain) in molecular biology laboratories for techniques such as agarose gel electrophoresis. It ...
(EtBr) is a popular nucleic acid stain. EtBr allows one to easily visualize DNA or RNA on a gel as EtBr fluoresces an orange color under UV light. Ethidium bromide binds nucleic acid chains through the process of Intercalation. While Ethidium bromide is a popular stain it is important to exercise caution when using EtBr as it is a known carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive sub ...
. Because of this fact, many researchers opt to use stains such as SYBR Green and SYBR Safe which are safer alternatives to EtBr. EtBr is used by simply adding it to the gel mixture. Once the gel has run, the gel may be viewed through the use of a photo-documentation system.
Silver staining is used when more sensitive method for detection is needed, as classical Coomassie Brilliant Blue staining can usually detect a 50 ng protein band, Silver staining increases the sensitivity typically 10-100 fold more. This is based on the chemistry of photographic development. The proteins are fixed to the gel with a dilute methanol solution, then incubated with an acidic silver nitrate solution. Silver ions are reduced to their metallic form by formaldehyde at alkaline pH. An acidic solution, such as acetic acid stops development. Silver staining was introduced by Kerenyi and Gallyas as a sensitive procedure to detect trace amounts of proteins in gel
A gel is a semi-solid that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state, although the liquid phase may still di ...
s. The technique has been extended to the study of other biological macromolecules
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
that have been separated in a variety of supports. Many variables can influence the colour
Color (American English) or colour (British English) is the visual perceptual property deriving from the spectrum of light interacting with the photoreceptor cells of the eyes. Color categories and physical specifications of color are associ ...
intensity and every protein has its own staining characteristics; clean glassware, pure reagents and water of highest purity are the key points to successful staining. Silver staining was developed in the 14th century for colouring the surface of glass. It has been used extensively for this purpose since the 16th century. The colour produced by the early silver stains ranged between light yellow and an orange-red. Camillo Golgi
Camillo Golgi (; 7 July 184321 January 1926) was an Italian biologist and pathologist known for his works on the central nervous system. He studied medicine at the University of Pavia (where he later spent most of his professional career) betwe ...
perfected the silver staining for the study of the nervous system
In biology, the nervous system is the highly complex part of an animal that coordinates its actions and sensory information by transmitting signals to and from different parts of its body. The nervous system detects environmental changes ...
. Golgi's method stains a limited number of cells at random in their entirety.
Autoradiography, also used for protein band detection post gel electrophoresis, uses radioactive isotopes to label proteins, which are then detected by using X-ray film.
Western blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
ting is a process by which proteins separated in the acrylamide gel are electrophoretically transferred to a stable, manipulable membrane such as a nitrocellulose
Nitrocellulose (also known as cellulose nitrate, flash paper, flash cotton, guncotton, pyroxylin and flash string, depending on form) is a highly flammable compound formed by nitrating cellulose through exposure to a mixture of nitric acid and ...
, nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic.
Nylon is a silk-like thermoplastic, generally made from pet ...
, or PVDF
Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) is a highly non-reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride.
PVDF is a specialty plastic used in applications requiring the highest pu ...
membrane. It is then possible to apply immunochemical techniques to visualise the transferred proteins, as well as accurately identify relative increases or decreases of the protein of interest.
See also
*Agarose gel electrophoresis
Agarose gel electrophoresis is a method of gel electrophoresis used in biochemistry, molecular biology, genetics, and clinical chemistry to separate a mixed population of macromolecules such as DNA or proteins in a matrix of agarose, one of the ...
*Capillary electrophoresis
Capillary electrophoresis (CE) is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. Very often, CE refers to capillary zone electrophoresis (CZE), but other electr ...
* DNA electrophoresis
*Eastern blotting
The eastern blot, or eastern blotting, is a biochemical technique used to analyze protein post-translational modifications including the addition of lipids, phosphates, and glycoconjugates. It is most often used to detect carbohydrate epitopes. Thu ...
*Electroblotting
Electroblotting is a method in molecular biology/biochemistry/immunogenetics to transfer proteins or nucleic acids onto a membrane by using PVDF or nitrocellulose, after gel electrophoresis. The protein or nucleic acid can then be further analyzed ...
*Fast parallel proteolysis (FASTpp)
Fast parallel proteolysis (FASTpp) is a method to determine the thermostability of proteins by measuring which fraction of protein resists rapid proteolytic digestion.
History and background
Proteolysis is widely used in biochemistry and cell ...
* History of electrophoresis
* Isoelectric focusing
* Isotachophoresis
* Native gel electrophoresis
*Northern blot
The northern blot, or RNA blot,Gilbert, S. F. (2000) Developmental Biology, 6th Ed. Sunderland MA, Sinauer Associates. is a technique used in molecular biology research to study gene expression by detection of RNA (or isolated mRNA) in a sampl ...
ting
*Protein electrophoresis
Protein electrophoresis is a method for analysing the proteins in a fluid or an extract. The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium: SDS polyacrylamide gel ...
*Southern blot
A Southern blot is a method used in molecular biology for detection of a specific DNA sequence in DNA samples. Southern blotting combines transfer of electrophoresis-separated DNA fragments to a filter membrane and subsequent fragment detecti ...
ting
* Two dimensional SDS-PAGE
* Zymography
References
External links
SDS-PAGE: How it Works
Demystifying SDS-PAGE
for customised recipes for TRIS Urea gels.
2-Dimensional Protein Gelelectrophoresis
Hempelmann E. SDS-Protein PAGE and Proteindetection by Silverstaining and Immunoblotting of Plasmodium falciparum proteins. in: Moll K, Ljungström J, Perlmann H, Scherf A, Wahlgren M (eds) Methods in Malaria Research, 5th edition, 2008, 263-266 {{DEFAULTSORT:Sds-Page Molecular biology Electrophoresis