Phases of fluorine on:
[Wikipedia]
[Google]
[Amazon]
__NOTOC__
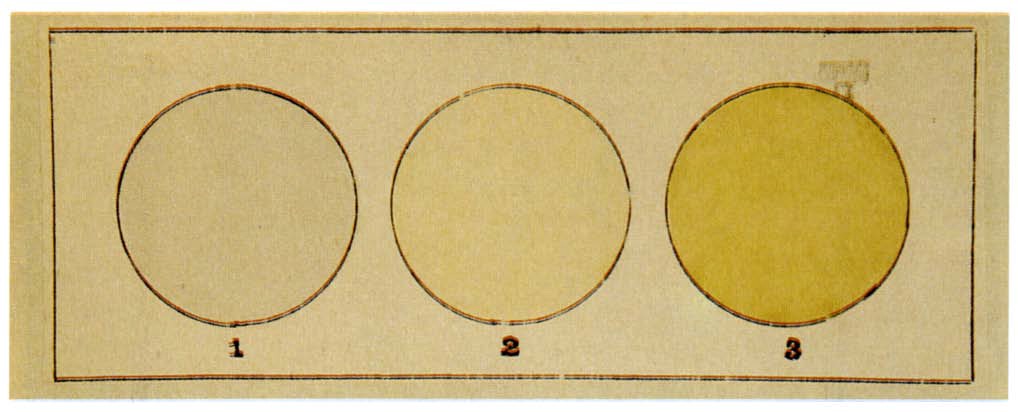
 Fluorine condenses to a bright yellow liquid at −188 °C (−307 °F), which is near the condensation temperatures of oxygen and nitrogen.
The solid state of fluorine relies on Van der Waals forces to hold molecules together, which, because of the small size of the fluorine molecules, are relatively weak. Consequently, the solid state of fluorine is more similar to that of oxygen or the noble gases than to those of the heavier halogens.
Fluorine condenses to a bright yellow liquid at −188 °C (−307 °F), which is near the condensation temperatures of oxygen and nitrogen.
The solid state of fluorine relies on Van der Waals forces to hold molecules together, which, because of the small size of the fluorine molecules, are relatively weak. Consequently, the solid state of fluorine is more similar to that of oxygen or the noble gases than to those of the heavier halogens.
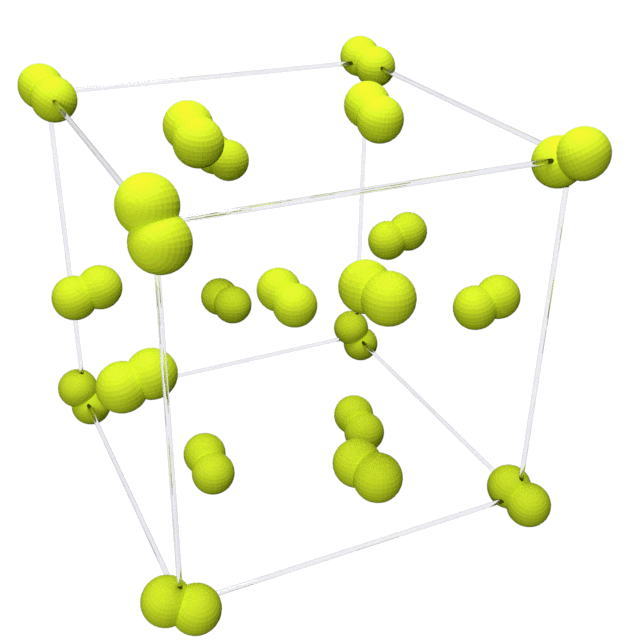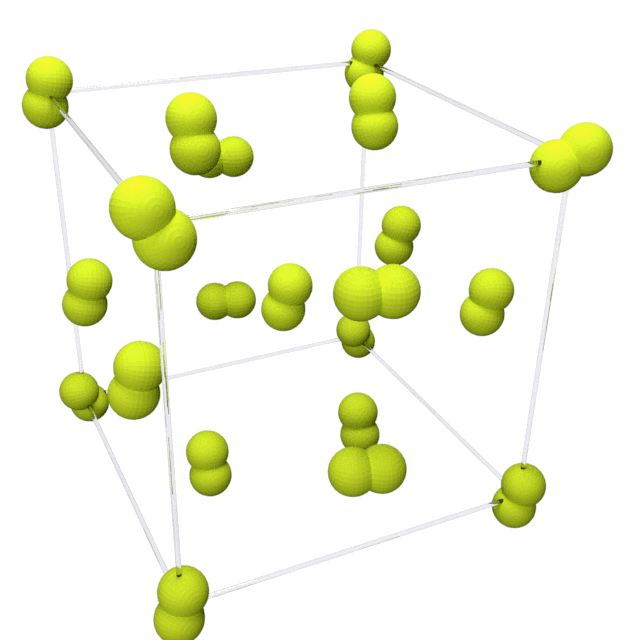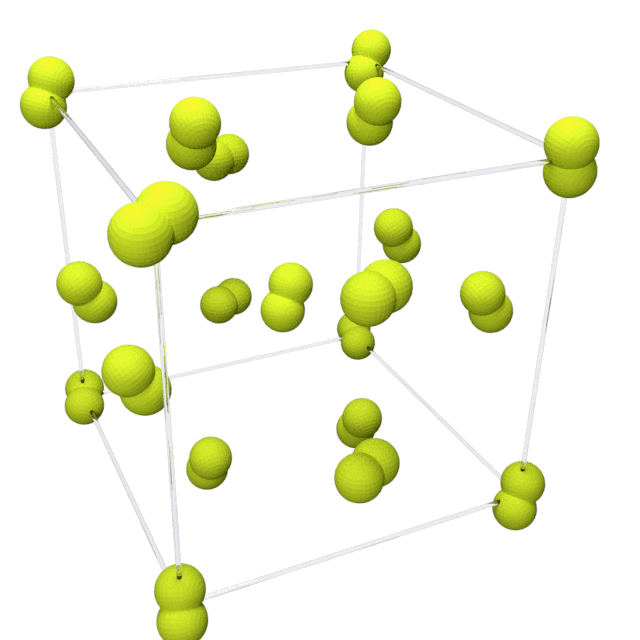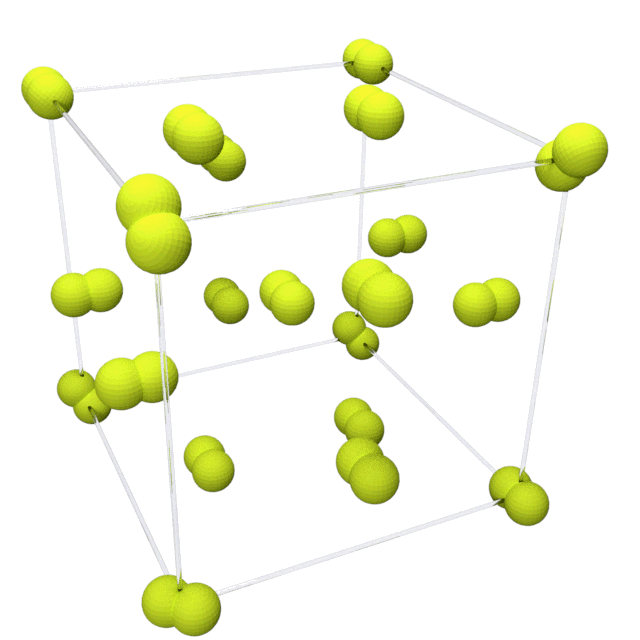 Fluorine solidifies at −220 °C (−363 °F) into a
Fluorine solidifies at −220 °C (−363 °F) into a  This phase is opaque and hard, with close-packed layers of molecules, and is denser at 1.97 g/cm3. The solid state phase change requires more energy than the melting point transition and can be violent, shattering samples and blowing out sample holder windows.
This phase is opaque and hard, with close-packed layers of molecules, and is denser at 1.97 g/cm3. The solid state phase change requires more energy than the melting point transition and can be violent, shattering samples and blowing out sample holder windows.
NASA ADS: Solid Fluorine and Solid Chlorine: Crystal Structures and Intermolecular Forces by S. C. Nyburg
Fluorine Phases of matter Allotropes

Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
forms diatomic molecules () that are gaseous at room temperature with a density about 1.3 times that of air. Though sometimes cited as yellow-green, pure fluorine gas is actually a very pale yellow. The color can only be observed in concentrated fluorine gas when looking down the axis of long tubes, as it appears transparent when observed from the side in normal tubes or if allowed to escape into the atmosphere. The element has a "pungent" characteristic odor that is noticeable in concentrations as low as 20 ppb.
 Fluorine condenses to a bright yellow liquid at −188 °C (−307 °F), which is near the condensation temperatures of oxygen and nitrogen.
The solid state of fluorine relies on Van der Waals forces to hold molecules together, which, because of the small size of the fluorine molecules, are relatively weak. Consequently, the solid state of fluorine is more similar to that of oxygen or the noble gases than to those of the heavier halogens.
Fluorine condenses to a bright yellow liquid at −188 °C (−307 °F), which is near the condensation temperatures of oxygen and nitrogen.
The solid state of fluorine relies on Van der Waals forces to hold molecules together, which, because of the small size of the fluorine molecules, are relatively weak. Consequently, the solid state of fluorine is more similar to that of oxygen or the noble gases than to those of the heavier halogens.
 Fluorine solidifies at −220 °C (−363 °F) into a
Fluorine solidifies at −220 °C (−363 °F) into a cubic
Cubic may refer to:
Science and mathematics
* Cube (algebra), "cubic" measurement
* Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex
** Cubic crystal system, a crystal system w ...
structure, called beta-fluorine. This phase
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
* Phase space, a mathematic ...
is transparent and soft, with significant disorder of the molecules; its density is 1.70 g/cm3. At −228 °C (−378 °F) fluorine undergoes a solid–solid phase transition into a monoclinic structure called alpha-fluorine.
 This phase is opaque and hard, with close-packed layers of molecules, and is denser at 1.97 g/cm3. The solid state phase change requires more energy than the melting point transition and can be violent, shattering samples and blowing out sample holder windows.
This phase is opaque and hard, with close-packed layers of molecules, and is denser at 1.97 g/cm3. The solid state phase change requires more energy than the melting point transition and can be violent, shattering samples and blowing out sample holder windows.
History
Henri Moissan was the first to isolate the element, observing its gaseous phase. Solid fluorine received significant study in the 1920s and 30s, but relatively less until the 1960s. The crystal structure of alpha-fluorine given, which still has some uncertainty, dates to a 1970 paper byLinus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific top ...
.
Notes
Citations
Indexed references
* * ** * * * * * * *Further reading
* * * *{{Cite journal , last1 = English , first1 = C. A. , last2 = Venables , first2 = J. A. , doi = 10.1098/rspa.1974.0140 , title = The Structure of the Diatomic Molecular Solids , journal = Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences , volume = 340 , issue = 1620 , pages = 57 , year = 1974 , bibcode = 1974RSPSA.340...57E , s2cid = 94525910 *http://www.osti.gov/bridge/servlets/purl/4010212-0BbwUC/4010212.pdf (phase diagrams of the elements) *http://jcp.aip.org/resource/1/jcpsa6/v47/i2/p740_s1?isAuthorized=no (sample holder blowout)NASA ADS: Solid Fluorine and Solid Chlorine: Crystal Structures and Intermolecular Forces by S. C. Nyburg
Fluorine Phases of matter Allotropes