Period 2 element on:
[Wikipedia]
[Google]
[Amazon]
A period 2 element is one of the
 Period 2 is the first period in the periodic table from which
Period 2 is the first period in the periodic table from which
 Lithium (Li) is an alkali metal with atomic number 3, occurring naturally in two isotopes: 6Li and 7Li. The two make up all natural occurrence of lithium on Earth, although further isotopes have been synthesized. In ionic compounds, lithium loses an
Lithium (Li) is an alkali metal with atomic number 3, occurring naturally in two isotopes: 6Li and 7Li. The two make up all natural occurrence of lithium on Earth, although further isotopes have been synthesized. In ionic compounds, lithium loses an
at WebElements. Lithium is one of the few elements synthesized in the Big Bang. Lithium is the 33rd most abundant element on earth, occurring in concentrations of between 20 and 70 ppm by weight, but due to its high reactivity it is only found naturally in compounds.Kamienski et al. "Lithium and lithium compounds". ''Kirk-Othmer Encyclopedia of Chemical Technology''. John Wiley & Sons, Inc. Published online 2004. Lithium
 Beryllium (Be) is the chemical element with atomic number 4, occurring in the form of 9Be. At standard temperature and pressure, beryllium is a strong, steel-grey, light-weight,
Beryllium (Be) is the chemical element with atomic number 4, occurring in the form of 9Be. At standard temperature and pressure, beryllium is a strong, steel-grey, light-weight,
at WebElements. It also has one of the highest
 Boron (B) is the chemical element with atomic number 5, occurring as 10B and 11B. At standard temperature and pressure, boron is a
Boron (B) is the chemical element with atomic number 5, occurring as 10B and 11B. At standard temperature and pressure, boron is a
at WebElements. Boron's most common
of boron. These are the only stable isotopes of boron; however other isotopes have been synthesised. Boron forms covalent bonds with other nonmetals and has
 Carbon is the chemical element with atomic number 6, occurring as 12C, 13C and 14C.Carbon
Carbon is the chemical element with atomic number 6, occurring as 12C, 13C and 14C.Carbon
at WebElements. At standard temperature and pressure, carbon is a solid, occurring in many different allotropes, the most common of which are
by Mahananda Dasgupta of the Department of Nuclear Physics at Australian National University. 13C is also stable, with six protons and seven neutrons, at 1.1%. Trace amounts of 14C also occur naturally but this isotope is radioactive and decays with a half life of 5730 years; it is used for radiocarbon dating. Other
 Nitrogen is the chemical element with atomic number 7, the symbol N and
Nitrogen is the chemical element with atomic number 7, the symbol N and
at WebElements. Many industrially important compounds, such as
 Fluorine is the chemical element with atomic number 9. It occurs naturally in its only stable form 19F.
Fluorine is a pale-yellow, diatomic gas under normal conditions and down to very low temperatures. Short one electron of the highly stable octet in each atom, fluorine molecules are unstable enough that they easily snap, with loose fluorine atoms tending to grab single electrons from just about any other element. Fluorine is the most reactive of all elements, and it even attacks many oxides to replace oxygen with fluorine. Fluorine even attacks silica, one of the favored materials for transporting strong acids, and burns asbestos. It attacks common salt, one of the most stable compounds, with the release of chlorine. It never appears uncombined in nature and almost never stays uncombined for long. It burns hydrogen simultaneously if either is liquid or gaseous—even at temperatures close to absolute zero. It is extremely difficult to isolate from any compounds, let alone keep uncombined.
Fluorine gas is extremely dangerous because it attacks almost all organic material, including live flesh. Many of the binary compounds that it forms (called fluorides) are themselves highly toxic, including soluble fluorides and especially hydrogen fluoride. Fluorine forms very strong bonds with many elements. With sulfur it can form the extremely stable and chemically inert sulfur hexafluoride; with carbon it can form the remarkable material
Fluorine is the chemical element with atomic number 9. It occurs naturally in its only stable form 19F.
Fluorine is a pale-yellow, diatomic gas under normal conditions and down to very low temperatures. Short one electron of the highly stable octet in each atom, fluorine molecules are unstable enough that they easily snap, with loose fluorine atoms tending to grab single electrons from just about any other element. Fluorine is the most reactive of all elements, and it even attacks many oxides to replace oxygen with fluorine. Fluorine even attacks silica, one of the favored materials for transporting strong acids, and burns asbestos. It attacks common salt, one of the most stable compounds, with the release of chlorine. It never appears uncombined in nature and almost never stays uncombined for long. It burns hydrogen simultaneously if either is liquid or gaseous—even at temperatures close to absolute zero. It is extremely difficult to isolate from any compounds, let alone keep uncombined.
Fluorine gas is extremely dangerous because it attacks almost all organic material, including live flesh. Many of the binary compounds that it forms (called fluorides) are themselves highly toxic, including soluble fluorides and especially hydrogen fluoride. Fluorine forms very strong bonds with many elements. With sulfur it can form the extremely stable and chemically inert sulfur hexafluoride; with carbon it can form the remarkable material
 Neon is the chemical element with atomic number 10, occurring as 20Ne, 21Ne and 22Ne.
Neon is a monatomic gas. With a complete octet of outer electrons it is highly resistant to removal of any electron, and it cannot accept an electron from anything. Neon has no tendency to form any normal compounds under normal temperatures and pressures; it is effectively inert. It is one of the so-called "noble gases".
Neon is a trace component of the atmosphere without any biological role.
Neon is the chemical element with atomic number 10, occurring as 20Ne, 21Ne and 22Ne.
Neon is a monatomic gas. With a complete octet of outer electrons it is highly resistant to removal of any electron, and it cannot accept an electron from anything. Neon has no tendency to form any normal compounds under normal temperatures and pressures; it is effectively inert. It is one of the so-called "noble gases".
Neon is a trace component of the atmosphere without any biological role.
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
s in the second row (or period
Period may refer to:
Common uses
* Era, a length or span of time
* Full stop (or period), a punctuation mark
Arts, entertainment, and media
* Period (music), a concept in musical composition
* Periodic sentence (or rhetorical period), a concept ...
) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
increases; a new row is started when chemical behavior begins to repeat, creating columns
A column or pillar in architecture and structural engineering is a structural element that transmits, through compression, the weight of the structure above to other structural elements below. In other words, a column is a compression membe ...
of elements with similar properties.
The second period contains the elements lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
, beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form m ...
, boron, carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, fluorine, and neon. In a quantum mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
description of atomic structure
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
, this period corresponds to the filling of the second () shell, more specifically its 2s and 2p subshells. Period 2 elements (carbon, nitrogen, oxygen, fluorine and neon) obey the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rul ...
in that they need eight electrons to complete their valence shell
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
(lithium and beryllium obey duet rule, boron is electron deficient.), where at most eight electrons can be accommodated: two in the 2s orbital and six in the 2p subshell.
Periodic trends
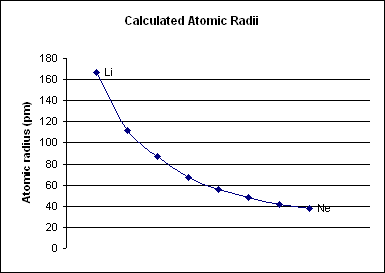
 Period 2 is the first period in the periodic table from which
Period 2 is the first period in the periodic table from which periodic trends
Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. They were discovered by the Russian chemist Dmitri Mendeleev in the year 1863. Major periodic trends include atom ...
can be drawn. Period 1, which only contains two elements (hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
and helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
), is too small to draw any conclusive trends from it, especially because the two elements behave nothing like other s-block elements. Period 2 has much more conclusive trends. For all elements in period 2, as the atomic number increases, the atomic radius
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there ...
of the elements decreases, the electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
increases, and the ionization energy increases.
Period 2 only has two metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
s (lithium and beryllium) of eight elements, less than for any subsequent period both by number and by proportion. It also has the most number of nonmetals, namely five, among all periods. The elements in period 2 often have the most extreme properties in their respective groups; for example, fluorine is the most reactive halogen, neon is the most inert noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
, and lithium is the least reactive alkali metal.
All period 2 elements completely obey the Madelung rule
The aufbau principle , from the German ''Aufbauprinzip'' (building-up principle), also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells ...
; in period 2, lithium and beryllium fill the 2s subshell, and boron, carbon, nitrogen, oxygen, fluorine, and neon fill the 2p subshell. The period shares this trait with periods 1 and 3, none of which contain transition element
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
s or inner transition element
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
s, which often vary from the rule.
:
Lithium
 Lithium (Li) is an alkali metal with atomic number 3, occurring naturally in two isotopes: 6Li and 7Li. The two make up all natural occurrence of lithium on Earth, although further isotopes have been synthesized. In ionic compounds, lithium loses an
Lithium (Li) is an alkali metal with atomic number 3, occurring naturally in two isotopes: 6Li and 7Li. The two make up all natural occurrence of lithium on Earth, although further isotopes have been synthesized. In ionic compounds, lithium loses an electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
to become positively charged, forming the cation Li+. Lithium is the first alkali metal in the periodic table,Hydrogen is occasionally referred to as an alkali metal, although this is rare. and the first metal of any kind in the periodic table.See note 1. At standard temperature and pressure, lithium is a soft, silver-white, highly reactive metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
. With a density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
of 0.564 g⋅cm−3, lithium is the lightest metal and the least dense solid element.Lithiumat WebElements. Lithium is one of the few elements synthesized in the Big Bang. Lithium is the 33rd most abundant element on earth, occurring in concentrations of between 20 and 70 ppm by weight, but due to its high reactivity it is only found naturally in compounds.Kamienski et al. "Lithium and lithium compounds". ''Kirk-Othmer Encyclopedia of Chemical Technology''. John Wiley & Sons, Inc. Published online 2004. Lithium
salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively c ...
are used in the pharmacology industry as mood stabilising drugs. They are used in the treatment of bipolar disorder
Bipolar disorder, previously known as manic depression, is a mental disorder characterized by periods of depression and periods of abnormally elevated mood that last from days to weeks each. If the elevated mood is severe or associated with ...
, where they have a role in treating depression and mania and may reduce the chances of suicide. The most common compounds used are lithium carbonate, Li2CO3, lithium citrate
Lithium citrate (Li3C6H5O7) is a chemical compound of lithium and citrate that is used as a mood stabilizer in psychiatric treatment of manic states and bipolar disorder. There is extensive pharmacology of lithium, the active component of this ...
, Li3C6H5O7, lithium sulphate, Li2SO4, and lithium orotate
Lithium orotate (C5H3LiN2O4) is a salt of orotic acid and lithium. It is available as the monohydrate, LiC5H3N2O4·H2O. In this compound, lithium is non-covalently bound to an orotate ion, rather than to a carbonate or other ion, and like other ...
, LiC5H3N2O4·H2O. Lithium is also used in batteries as an anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic ...
and its alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s with aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
and manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
are used to make high performance parts for aircraft
An aircraft is a vehicle that is able to fly by gaining support from the air. It counters the force of gravity by using either static lift or by using the dynamic lift of an airfoil, or in a few cases the downward thrust from jet engine ...
, most notably the external tank
The Space Shuttle external tank (ET) was the component of the Space Shuttle launch vehicle that contained the liquid hydrogen fuel and liquid oxygen oxidizer. During lift-off and ascent it supplied the fuel and oxidizer under pressure to ...
of the Space Shuttle
The Space Shuttle is a retired, partially reusable low Earth orbital spacecraft system operated from 1981 to 2011 by the U.S. National Aeronautics and Space Administration (NASA) as part of the Space Shuttle program. Its official program ...
.
Beryllium
 Beryllium (Be) is the chemical element with atomic number 4, occurring in the form of 9Be. At standard temperature and pressure, beryllium is a strong, steel-grey, light-weight,
Beryllium (Be) is the chemical element with atomic number 4, occurring in the form of 9Be. At standard temperature and pressure, beryllium is a strong, steel-grey, light-weight, brittle
A material is brittle if, when subjected to stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. Br ...
, bivalent alkali earth metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all ...
, with a density of 1.85 g⋅cm−3.Berylliumat WebElements. It also has one of the highest
melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
s of all the light metal
A light metal is any metal of relatively low density. More specific definitions have been proposed; none have obtained widespread acceptance. Magnesium, aluminium and titanium are light metals of significant commercial importance. Their densities ...
s. Beryllium's most common isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
is 9Be, which contains 4 protons and 5 neutrons. It makes up almost 100% of all naturally occurring beryllium and is its only stable isotope; however other isotopes have been synthesised. In ionic compounds, beryllium loses its two valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair form ...
s to form the cation, Be2+.
Small amounts of beryllium were synthesised during the Big Bang, although most of it decayed or reacted further to create larger nuclei, like carbon, nitrogen or oxygen. Beryllium is a component of 100 out of 4000 known mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2 ...
s, such as bertrandite
Bertrandite is a beryllium sorosilicate hydroxide mineral with composition: Be4Si2O7(OH)2. Bertrandite is a colorless to pale yellow orthorhombic mineral with a hardness of 6-7.
It is commonly found in beryllium rich pegmatites and is in part an ...
, Be4Si2O7(OH)2, beryl
Beryl ( ) is a mineral composed of beryllium aluminium silicate with the chemical formula Be3Al2Si6O18. Well-known varieties of beryl include emerald and aquamarine. Naturally occurring, hexagonal crystals of beryl can be up to several ...
, Al2Be3Si6O18, chrysoberyl
The mineral or gemstone chrysoberyl is an aluminate of beryllium with the formula Be Al2 O4. The name chrysoberyl is derived from the Greek words χρυσός ''chrysos'' and βήρυλλος ''beryllos'', meaning "a gold-white spar". Despite ...
, Al2BeO4, and phenakite
Phenakite or phenacite is a fairly rare nesosilicate mineral consisting of beryllium orthosilicate, Be2 Si O4. Occasionally used as a gemstone, phenakite occurs as isolated crystals, which are rhombohedral with parallel-faced hemihedrism, and are ...
, Be2SiO4. Precious forms of beryl are aquamarine, red beryl
Red beryl, formerly known as bixbite and marketed as red emerald or scarlet emerald, is an extremely rare variety of beryl as well as one of the rarest minerals on Earth. The gem gets its red color from manganese ions embedded inside of beryllium ...
and emerald. The most common sources of beryllium used commercially are beryl and bertrandite and production of it involves the reduction of beryllium fluoride
Beryllium fluoride is the inorganic compound with the formula Be F2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.
Properties
B ...
with magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
metal or the electrolysis of molten beryllium chloride
Beryllium chloride is an inorganic compound with the formula BeCl2. It is a colourless, hygroscopic solid that dissolves well in many polar solvents. Its properties are similar to those of aluminium chloride, due to beryllium's diagonal relatio ...
, containing some sodium chloride as beryllium chloride is a poor conductor of electricity.
Due to its stiffness, light weight, and dimensional stability over a wide temperature range, beryllium metal is used in as a structural material in aircraft, missiles and communication satellite
A communications satellite is an artificial satellite that relays and amplifies radio telecommunication signals via a transponder; it creates a communication channel between a source transmitter and a receiver at different locations on Earth. C ...
s. It is used as an alloying agent in beryllium copper, which is used to make electrical components due to its high electrical and heat conductivity. Sheets of beryllium are used in X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
detectors to filter out visible light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
and let only X-rays through. It is used as a neutron moderator in nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s because light nuclei are more effective at slowing down neutrons than heavy nuclei. Beryllium's low weight and high rigidity also make it useful in the construction of tweeter
A tweeter or treble speaker is a special type of loudspeaker (usually dome, inverse dome or horn-type) that is designed to produce high audio frequencies, typically deliver high frequencies up to 100 kHz. The name is derived from the high ...
s in loudspeaker
A loudspeaker (commonly referred to as a speaker or speaker driver) is an electroacoustic transducer that converts an electrical audio signal into a corresponding sound. A ''speaker system'', also often simply referred to as a "speaker" or ...
s.
Beryllium and beryllium compounds are classified by the International Agency for Research on Cancer
The International Agency for Research on Cancer (IARC; french: Centre International de Recherche sur le Cancer, CIRC) is an intergovernmental agency forming part of the World Health Organization of the United Nations.
Its role is to conduct and ...
as Group 1 carcinogens; they are carcinogenic to both animals and humans. Chronic berylliosis
Berylliosis, or chronic beryllium disease (CBD), is a chronic allergic-type lung response and chronic lung disease caused by exposure to beryllium and its compounds, a form of beryllium poisoning. It is distinct from acute beryllium poisoning, wh ...
is a pulmonary
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of ...
and systemic granulomatous
A granuloma is an aggregation of macrophages that forms in response to chronic inflammation. This occurs when the immune system attempts to isolate foreign substances that it is otherwise unable to eliminate. Such substances include infectiou ...
disease caused by exposure to beryllium. Between 1% – 15% of people are sensitive to beryllium and may develop an inflammatory reaction in their respiratory system
The respiratory system (also respiratory apparatus, ventilatory system) is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies ...
and skin
Skin is the layer of usually soft, flexible outer tissue covering the body of a vertebrate animal, with three main functions: protection, regulation, and sensation.
Other animal coverings, such as the arthropod exoskeleton, have different de ...
, called chronic beryllium disease or berylliosis
Berylliosis, or chronic beryllium disease (CBD), is a chronic allergic-type lung response and chronic lung disease caused by exposure to beryllium and its compounds, a form of beryllium poisoning. It is distinct from acute beryllium poisoning, wh ...
. The body's immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splint ...
recognises the beryllium as foreign particles and mounts an attack against them, usually in the lungs where they are breathed in. This can cause fever, fatigue, weakness, night sweats and difficulty in breathing.
Boron
 Boron (B) is the chemical element with atomic number 5, occurring as 10B and 11B. At standard temperature and pressure, boron is a
Boron (B) is the chemical element with atomic number 5, occurring as 10B and 11B. At standard temperature and pressure, boron is a trivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
metalloid that has several different allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
. Amorphous boron is a brown powder formed as a product of many chemical reactions. Crystalline
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
boron is a very hard, black material with a high melting point and exists in many polymorphs: Two rhombohedral
In geometry, a rhombohedron (also called a rhombic hexahedron or, inaccurately, a rhomboid) is a three-dimensional figure with six faces which are rhombi. It is a special case of a parallelepiped where all edges are the same length. It can be us ...
forms, α-boron and β-boron containing 12 and 106.7 atoms in the rhombohedral unit cell respectively, and 50-atom tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a squar ...
boron are the most common. Boron has a density of 2.34−3. Boronat WebElements. Boron's most common
isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
is 11B at 80.22%, which contains 5 protons and 6 neutrons. The other common isotope is 10B at 19.78%, which contains 5 protons and 5 neutrons.Propertiesof boron. These are the only stable isotopes of boron; however other isotopes have been synthesised. Boron forms covalent bonds with other nonmetals and has
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s of 1, 2, 3 and 4.
Boron does not occur naturally as a free element, but in compounds such as borate
A borate is any of several boron oxyanions, negative ions consisting of boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt with such anions, such as sodium metaborate, and disodium tetraborate . The name also re ...
s. The most common sources of boron are tourmaline
Tourmaline ( ) is a crystalline silicate mineral group in which boron is compounded with elements such as aluminium, iron, magnesium, sodium, lithium, or potassium. Tourmaline is a gemstone and can be found in a wide variety of colors.
The te ...
, borax
Borax is a salt ( ionic compound), a hydrated borate of sodium, with chemical formula often written . It is a colorless crystalline solid, that dissolves in water to make a basic solution. It is commonly available in powder or granular for ...
, Na2B4O5(OH)4·8H2O, and kernite, Na2B4O5(OH)4·2H2O. it is difficult to obtain pure boron. It can be made through the magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
reduction of boron trioxide
Boron trioxide or diboron trioxide is the oxide of boron with the formula . It is a colorless transparent solid, almost always glassy (amorphous), which can be crystallized only with great difficulty. It is also called boric oxide or boria. It h ...
, B2O3. This oxide is made by melting boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolve ...
, B(OH)3, which in turn is obtained from borax. Small amounts of pure boron can be made by the thermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is re ...
of boron bromide, BBr3, in hydrogen gas over hot tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that ...
wire, which acts as a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. The most commercially important sources of boron are: sodium tetraborate pentahydrate, Na2B4O7 · 5H2O, which is used in large amounts in making insulating fiberglass
Fiberglass (American English) or fibreglass ( Commonwealth English) is a common type of fiber-reinforced plastic using glass fiber. The fibers may be randomly arranged, flattened into a sheet called a chopped strand mat, or woven into glass clo ...
and sodium perborate
Sodium perborate is chemical compound whose chemical formula may be written , , or, more properly, ·. Its name is sometimes abbreviated as PBS (not to be confused with phosphate-buffered saline).
The compound is commonly encountered in anhydr ...
bleach; boron carbide, a ceramic
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain ...
material, is used to make armour materials, especially in bulletproof vests for soldiers and police officers; orthoboric acid, H3BO3 or boric acid, used in the production of textile fiberglass
Fiberglass (American English) or fibreglass ( Commonwealth English) is a common type of fiber-reinforced plastic using glass fiber. The fibers may be randomly arranged, flattened into a sheet called a chopped strand mat, or woven into glass clo ...
and flat panel display
A flat-panel display (FPD) is an electronic display used to display visual content such as text or images. It is present in consumer, medical, transportation, and industrial equipment.
Flat-panel displays are thin, lightweight, provide better l ...
s; sodium tetraborate decahydrate, Na2B4O7 · 10H2O or borax, used in the production of adhesives; and the isotope boron-10 is used as a control for nuclear reactors, as a shield for nuclear radiation, and in instruments used for detecting neutrons.
Boron is an essential plant micronutrient, required for cell wall strength and development, cell division, seed and fruit development, sugar transport and hormone development. However, high soil concentrations of over 1.0 ppm can cause necrosis in leaves and poor growth. Levels as low as 0.8 ppm can cause these symptoms to appear in plants particularly boron-sensitive. Most plants, even those tolerant of boron in the soil, will show symptoms of boron toxicity when boron levels are higher than 1.8 ppm. In animals, boron is an ultratrace element; in human diets, daily intake ranges from 2.1 to 4.3 mg boron/kg body weight (bw)/day. It is also used as a supplement for the prevention and treatment of osteoporosis and arthritis.
Carbon
 Carbon is the chemical element with atomic number 6, occurring as 12C, 13C and 14C.Carbon
Carbon is the chemical element with atomic number 6, occurring as 12C, 13C and 14C.Carbonat WebElements. At standard temperature and pressure, carbon is a solid, occurring in many different allotropes, the most common of which are
graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on lar ...
, diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, ...
, the fullerenes
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
and amorphous carbon Amorphous carbon is free, reactive carbon that has no crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amor ...
. Graphite is a soft, hexagonal crystalline, opaque black semimetal
A semimetal is a material with a very small overlap between the bottom of the conduction band and the top of the valence band.
According to electronic band theory, solids can be classified as insulators, semiconductors, semimetals, or metals ...
with very good conductive
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. Electric current is gene ...
and thermodynamically stable properties. Diamond however is a highly transparent
Transparency, transparence or transparent most often refer to:
* Transparency (optics), the physical property of allowing the transmission of light through a material
They may also refer to:
Literal uses
* Transparency (photography), a still, ...
colourless cubic crystal with poor conductive properties, is the hardest known naturally occurring mineral and has the highest refractive index
In optics, the refractive index (or refraction index) of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium.
The refractive index determines how much the path of light is bent, or ...
of all gemstones
A gemstone (also called a fine gem, jewel, precious stone, or semiprecious stone) is a piece of mineral crystal which, in cut and polished form, is used to make jewelry or other adornments. However, certain rocks (such as lapis lazuli, opal, ...
. In contrast to the crystal lattice structure of diamond and graphite, the fullerenes
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
are molecules, named after Richard Buckminster Fuller
Richard Buckminster Fuller (; July 12, 1895 – July 1, 1983) was an American people, American architect, systems theorist, writer, designer, inventor, philosopher, and futurist. He styled his name as R. Buckminster Fuller in his writings, ...
whose architecture the molecules resemble. There are several different fullerenes, the most widely known being the "buckeyball" C60. Little is known about the fullerenes and they are a current subject of research. There is also amorphous carbon, which is carbon without any crystalline structure. In mineralogy, the term is used to refer to soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolysed ...
and coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when ...
, although these are not truly amorphous as they contain small amounts of graphite or diamond. Carbon's most common isotope at 98.9% is 12C, with six protons and six neutrons.Presentation about isotopesby Mahananda Dasgupta of the Department of Nuclear Physics at Australian National University. 13C is also stable, with six protons and seven neutrons, at 1.1%. Trace amounts of 14C also occur naturally but this isotope is radioactive and decays with a half life of 5730 years; it is used for radiocarbon dating. Other
isotopes of carbon
Carbon (6C) has 15 known isotopes, from to , of which and are stable. The longest-lived radioisotope is , with a half-life of years. This is also the only carbon radioisotope found in nature—trace quantities are formed cosmogenically by t ...
have also been synthesised. Carbon forms covalent bonds with other non-metals with an oxidation state of −4, −2, +2 or +4.
Carbon is the fourth most abundant element in the universe by mass after hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
, helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
and oxygen and is the second most abundant element in the human body by mass after oxygen, the third most abundant by number of atoms. There are an almost infinite number of compounds that contain carbon due to carbon's ability to form long stable chains of C — C bonds. The simplest carbon-containing molecules are the hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s, which contain carbon and hydrogen, although they sometimes contain other elements in functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s. Hydrocarbons are used as fossil fuels and to manufacture plastics and petrochemicals. All organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s, those essential for life, contain at least one atom of carbon. When combined with oxygen and hydrogen, carbon can form many groups of important biological compounds including sugars, lignan
The lignans are a large group of low molecular weight polyphenols found in plants, particularly seeds, whole grains, and vegetables. The name derives from the Latin word for "wood". Lignans are precursors to phytoestrogens. They may play a role ...
s, chitins, alcohols, fat
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.
The term often refers specifically to triglycerides (triple est ...
s, and aromatic ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
s, carotenoids
Carotenoids (), also called tetraterpenoids, are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, co ...
and terpenes
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ar ...
. With nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
it forms alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...
s, and with the addition of sulfur also it forms antibiotics, amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s, and rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, an ...
products. With the addition of phosphorus to these other elements, it forms DNA and RNA, the chemical-code carriers of life, and adenosine triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms o ...
(ATP), the most important energy-transfer molecule in all living cells.
Nitrogen
 Nitrogen is the chemical element with atomic number 7, the symbol N and
Nitrogen is the chemical element with atomic number 7, the symbol N and atomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&nb ...
14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless and mostly inert diatomic gas at standard conditions
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union ...
, constituting 78.08% by volume of Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing fo ...
. The element nitrogen was discovered as a separable component of air, by Scottish physician Daniel Rutherford
Daniel Rutherford (3 November 1749 – 15 December 1819) was a Scottish physician, chemist and botanist who is known for the isolation of nitrogen in 1772.
Life
Rutherford was born on 3 November 1749, the son of Anne Mackay and Professor John ...
, in 1772. It occurs naturally in form of two isotopes: nitrogen-14 and nitrogen-15.Nitrogenat WebElements. Many industrially important compounds, such as
ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
, nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
, organic nitrates ( propellants and explosives), and cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
s, contain nitrogen. The extremely strong bond in elemental nitrogen dominates nitrogen chemistry, causing difficulty for both organisms and industry in breaking the bond to convert the molecule into useful compounds, but at the same time causing release of large amounts of often useful energy when the compounds burn, explode, or decay back into nitrogen gas.
Nitrogen occurs in all living organisms, and the nitrogen cycle describes movement of the element from air into the biosphere
The biosphere (from Greek βίος ''bíos'' "life" and σφαῖρα ''sphaira'' "sphere"), also known as the ecosphere (from Greek οἶκος ''oîkos'' "environment" and σφαῖρα), is the worldwide sum of all ecosystems. It can also ...
and organic compounds, then back into the atmosphere. Synthetically produced nitrates are key ingredients of industrial fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
s, and also key pollutants in causing the eutrophication
Eutrophication is the process by which an entire body of water, or parts of it, becomes progressively enriched with minerals and nutrients, particularly nitrogen and phosphorus. It has also been defined as "nutrient-induced increase in phytopla ...
of water systems. Nitrogen is a constituent element of amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s and thus of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s, and of nucleic acids ( DNA and RNA). It resides in the chemical structure
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of ...
of almost all neurotransmitters, and is a defining component of alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...
s, biological molecules produced by many organisms.
Oxygen
Oxygen is the chemical element with atomic number 8, occurring mostly as 16O, but also 17O and 18O. Oxygen is the third-most common element by mass in the universe (although there are more carbon atoms, each carbon atom is lighter). It is highly electronegative and non-metallic, usually diatomic, gas down to very low temperatures. Only fluorine is more reactive among non-metallic elements. It is two electrons short of a full octet and readily takes electrons from other elements. It reacts violently withalkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
and white phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
White phosphorus
White ...
at room temperature and less violently with alkali earth metals heavier than magnesium. At higher temperatures it burns most other metals and many non-metals (including hydrogen, carbon, and sulfur). Many oxides are extremely stable substances difficult to decompose—like water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
, carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
, alumina, silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is ...
, and iron oxides (the latter often appearing as rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO( ...
). Oxygen is part of substances best described as some salts of metals and oxygen-containing acids (thus nitrates, sulfates, phosphates, silicates, and carbonates.
Oxygen is essential to all life. Plants and phytoplankton photosynthesize water and carbon dioxide and water, both oxides, in the presence of sunlight to form sugars with the release of oxygen. The sugars are then turned into such substances as cellulose and (with nitrogen and often sulfur) proteins and other essential substances of life. Animals especially but also fungi and bacteria ultimately depend upon photosynthesizing plants and phytoplankton for food and oxygen.
Fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction products.
At a certain point in the combustion reaction, called the ignition point, flames a ...
uses oxygen to oxidize compounds typically of carbon and hydrogen to water and carbon dioxide (although other elements may be involved) whether in uncontrolled conflagrations that destroy buildings and forests or the controlled fire within engines or that supply electrical energy from turbines, heat for keeping buildings warm, or the motive force that drives vehicles.
Oxygen forms roughly 21% of the Earth's atmosphere; all of this oxygen is the result of photosynthesis. Pure oxygen has use in medical treatment of people who have respiratory difficulties. Excess oxygen is toxic.
Oxygen was originally associated with the formation of acids—until some acids were shown to not have oxygen in them. Oxygen is named for its formation of acids, especially with non-metals. Some oxides of some non-metals are extremely acidic, like sulfur trioxide, which forms sulfuric acid on contact with water. Most oxides with metals are alkaline, some extremely so, like potassium oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertili ...
. Some metallic oxides are amphoteric, like aluminum oxide, which means that they can react with both acids and bases.
Although oxygen is normally a diatomic gas, oxygen can form an allotrope known as ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
. Ozone is a triatomic gas even more reactive than oxygen. Unlike regular diatomic oxygen, ozone is a toxic material generally considered a pollutant. In the upper atmosphere, some oxygen forms ozone which has the property of absorbing dangerous ultraviolet rays within the ozone layer. Land life was impossible before the formation of an ozone layer.
Fluorine
 Fluorine is the chemical element with atomic number 9. It occurs naturally in its only stable form 19F.
Fluorine is a pale-yellow, diatomic gas under normal conditions and down to very low temperatures. Short one electron of the highly stable octet in each atom, fluorine molecules are unstable enough that they easily snap, with loose fluorine atoms tending to grab single electrons from just about any other element. Fluorine is the most reactive of all elements, and it even attacks many oxides to replace oxygen with fluorine. Fluorine even attacks silica, one of the favored materials for transporting strong acids, and burns asbestos. It attacks common salt, one of the most stable compounds, with the release of chlorine. It never appears uncombined in nature and almost never stays uncombined for long. It burns hydrogen simultaneously if either is liquid or gaseous—even at temperatures close to absolute zero. It is extremely difficult to isolate from any compounds, let alone keep uncombined.
Fluorine gas is extremely dangerous because it attacks almost all organic material, including live flesh. Many of the binary compounds that it forms (called fluorides) are themselves highly toxic, including soluble fluorides and especially hydrogen fluoride. Fluorine forms very strong bonds with many elements. With sulfur it can form the extremely stable and chemically inert sulfur hexafluoride; with carbon it can form the remarkable material
Fluorine is the chemical element with atomic number 9. It occurs naturally in its only stable form 19F.
Fluorine is a pale-yellow, diatomic gas under normal conditions and down to very low temperatures. Short one electron of the highly stable octet in each atom, fluorine molecules are unstable enough that they easily snap, with loose fluorine atoms tending to grab single electrons from just about any other element. Fluorine is the most reactive of all elements, and it even attacks many oxides to replace oxygen with fluorine. Fluorine even attacks silica, one of the favored materials for transporting strong acids, and burns asbestos. It attacks common salt, one of the most stable compounds, with the release of chlorine. It never appears uncombined in nature and almost never stays uncombined for long. It burns hydrogen simultaneously if either is liquid or gaseous—even at temperatures close to absolute zero. It is extremely difficult to isolate from any compounds, let alone keep uncombined.
Fluorine gas is extremely dangerous because it attacks almost all organic material, including live flesh. Many of the binary compounds that it forms (called fluorides) are themselves highly toxic, including soluble fluorides and especially hydrogen fluoride. Fluorine forms very strong bonds with many elements. With sulfur it can form the extremely stable and chemically inert sulfur hexafluoride; with carbon it can form the remarkable material Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemo ...
that is a stable and non-combustible solid with a high melting point and a very low coefficient of friction that makes it an excellent liner for cooking pans and raincoats. Fluorine-carbon compounds include some unique plastics.
it is also used as a reactant in the making of toothpaste.
Neon
 Neon is the chemical element with atomic number 10, occurring as 20Ne, 21Ne and 22Ne.
Neon is a monatomic gas. With a complete octet of outer electrons it is highly resistant to removal of any electron, and it cannot accept an electron from anything. Neon has no tendency to form any normal compounds under normal temperatures and pressures; it is effectively inert. It is one of the so-called "noble gases".
Neon is a trace component of the atmosphere without any biological role.
Neon is the chemical element with atomic number 10, occurring as 20Ne, 21Ne and 22Ne.
Neon is a monatomic gas. With a complete octet of outer electrons it is highly resistant to removal of any electron, and it cannot accept an electron from anything. Neon has no tendency to form any normal compounds under normal temperatures and pressures; it is effectively inert. It is one of the so-called "noble gases".
Neon is a trace component of the atmosphere without any biological role.
Notes
References
External links
* {{DEFAULTSORT:Period 02 Periods (periodic table) Pages containing element color directly