P2O5 on:
[Wikipedia]
[Google]
[Amazon]
Phosphorus pentoxide is a
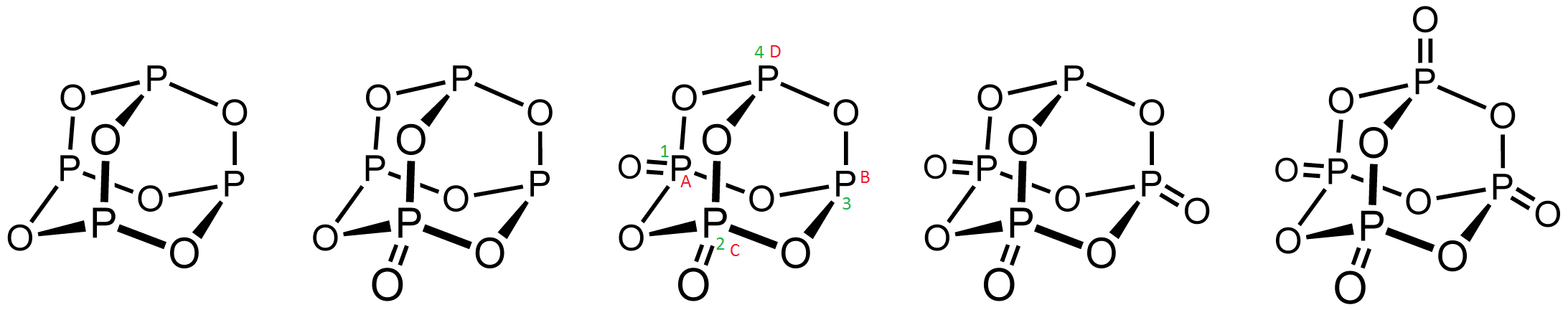 On observation it will be seen that double bonded oxygen in
On observation it will be seen that double bonded oxygen in P4O8 at 1,2 position or 1,3 position are identical and both positions have same steric hindrance. Cycle 12341 and ABCDA are identical.
Phosphorus pentoxide MSDS
/ref>
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with molecular formula P4 O10 (with its common name derived from its empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the ...
, P2O5). This white crystalline solid is the anhydride
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the pa ...
of phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, w ...
. It is a powerful desiccant and dehydrating agent.
Structure
Phosphorus pentoxide crystallizes in at least four forms or polymorphs. The most familiar one, a metastable form (shown in the figure), comprises molecules of P4O10. Weakvan der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s hold these molecules together in a hexagonal lattice (However, in spite of the high symmetry of the molecules, the crystal packing is not a close packing). The structure of the P4O10 cage is reminiscent of adamantane
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the ...
with ''T''d symmetry point group. It is closely related to the corresponding anhydride of phosphorous acid
Phosphorous acid (or phosphonic acid (singular)) is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in th ...
, P4O6. The latter lacks terminal oxo groups. Its density is 2.30 g/cm3. It boils at 423 °C under atmospheric pressure; if heated more rapidly it can sublimate. This form can be made by condensing the vapor of phosphorus pentoxide rapidly, and the result is an extremely hygroscopic solid..
The other polymorphs are polymeric, but in each case the phosphorus atoms are bound by a tetrahedron of oxygen atoms, one of which forms a terminal P=O bond involving the donation of the terminal oxygen p-orbital electrons to the antibonding phosphorus-oxygen single bonds. The macromolecular form can be made by heating the compound in a sealed tube for several hours, and maintaining the melt at a high temperature before cooling the melt to the solid. The metastable orthorhombic "O"-form (density 2.72 g/cm3, melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
562 °C) adopts a layered structure consisting of interconnected P6O6 rings, not unlike the structure adopted by certain poly silicates. The stable form is a higher density phase, also orthorhombic, the so-called O' form. It consists of a 3-dimensional framework, density 3.5 g/cm3. The remaining polymorph is a glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling ( quenching ...
or amorphous form; it can be made by fusing any of the others.
Preparation
P4O10 is prepared by burningtetraphosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
White phosphorus
White ...
with sufficient supply of oxygen:
: P4 + 5 O2 → P4O10
For most of the 20th century, phosphorus pentoxide was used to provide a supply of concentrated pure phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, w ...
. In the thermal process, the phosphorus pentoxide obtained by burning white phosphorus was dissolved in dilute phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, w ...
to produce concentrated acid. Improvements in filter technology is leading to the "wet phosphoric acid process" taking over from the thermal process, obviating the need to produce white phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
White phosphorus
White ...
as a starting material. The dehydration of phosphoric acid to give phosphorus pentoxide is not possible as on heating metaphosphoric acid will boil without losing all its water.
Applications
Phosphorus pentoxide is a potentdehydrating
In physiology, dehydration is a lack of total body water, with an accompanying disruption of Metabolism, metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental tempe ...
agent as indicated by the exothermic nature of its hydrolysis producing phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, w ...
:
:P4O10 + 6 H2O → 4 H3PO4 (–177 kJ)
However, its utility for drying is limited somewhat by its tendency to form a protective viscous coating that inhibits further dehydration by unspent material. A granular form of P4O10 is used in desiccator
Desiccators are sealable enclosures containing desiccants used for preserving moisture-sensitive items such as cobalt chloride paper for another use. A common use for desiccators is to protect chemicals which are hygroscopic or which react with w ...
s.
Consistent with its strong desiccating power, P4O10 is used in organic synthesis for dehydration. The most important application is for the conversion of primary amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
s into nitriles
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
:
:P4O10 + RC(O)NH2 → P4O9(OH)2 + RCN
The indicated coproduct P4O9(OH)2 is an idealized formula for undefined products resulting from the hydration of P4O10.
Alternatively, when combined with a carboxylic acid, the result is the corresponding anhydride
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the pa ...
:
:P4O10 + RCO2H → P4O9(OH)2 + C(O)sub>2O
The "Onodera reagent", a solution of P4O10 in DMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds ...
, is employed for the oxidation of alcohols. This reaction is reminiscent of the Swern oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one ...
.
The desiccating power of P4O10 is strong enough to convert many mineral acids to their anhydrides. Examples: HNO3 is converted to N2O5; H2SO4 is converted to SO3; HClO4 is converted to Cl2O7; CF3SO3H is converted to (CF3)2S2O5.
Agriculture
The compound can be used as cropfertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
.
Related phosphorus oxides
Between the commercially important P4O6 and P4O10, phosphorus oxides are known with intermediate structures. On observation it will be seen that double bonded oxygen in
On observation it will be seen that double bonded oxygen in Hazards
Phosphorus pentoxide itself is not flammable. Just like sulfur trioxide, it reacts vigorously with water and water-containing substances like wood or cotton, liberates much heat and may even cause fire due to the highly exothermic nature of such reactions. It is corrosive to metal and is very irritating – it may cause severe burns to the eye, skin,mucous membrane
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It i ...
, and respiratory tract even at concentrations as low as 1 mg/m3./ref>
See also
*Eaton's reagent Eaton's reagent (10 wt% phosphorus pentoxide solution in methanesulfonic acid) is used as an alternative to polyphosphoric acid in chemical synthesis to promote acylation
In chemistry, acylation (or alkanoylation) is the chemical reaction in whi ...
References
External links
{{Oxides Inorganic phosphorus compounds Acid anhydrides Acidic oxides Glass compositions Dehydrating agents Adamantane-like molecules Phosphorus oxides Phosphorus(V) compounds Deliquescent substances